The Role of Nanomaterials and Macrophage Polarization in Inflammation and Cancer Treatment
Author'(s): Ashok Chakraborty Ph.D* and Anil Diwan Ph.D
AllExcel, Inc., Shelton, CT, USA
*Correspondence:
Ashok Chakraborty, AllExcel, Inc., Shelton, CT, USA.
Received: 17 Jul 2022; Accepted: 25 Aug 2022; Published: 30 Aug 2022
Citation: Chakraborty A, Diwan A. The Role of Nanomaterials and Macrophage Polarization in Inflammation and Cancer Treatment.Cancer Sci Res. 2022; 5(3): 1-8.
Abstract
Macrophages (MΦs) are functionally plastic and can rapidly adopt different phenotypes based on the stimuli they received from their surroundings. For instance, (MΦs) can differentiate into pro-inflammatory M1-type secreting various pro-inflammatory cytokines, e.g., IL-6, IL-12 and TNF-α. This is an essential defensive mechanism against invading pathogens, but also contributes to tissue destruction. On the other hand, M2-type MΦs have immunosuppressive activity, produces high level of anti-inflammatory cytokine IL-10 and mediates tissue repair. Their unique plasticity and the balance in between different phenotypes maintain the immune homeostasis in the lung under healthy conditions. The role of these two forms of MΦs are also evident in cancer biology. While tissue resident MΦs those who are of M1 type are exerting the immune challenge to the tumor cells, the M2 type from the tumor microenvironment (TME) are supporting the growth and progression of cancer cells.
Nanodevices are thought to be an ideal agent targeting macrophages owing to their intrinsic nanoscale property suitable for phagocytosis. The induction of MΦs polarization has been employed in various studies, where administration of IL-4 could reprogram the endogenous inflammatory macrophages (M1-type) to anti-inflammatory ones (M2-type), and accelerate resolution of inflammation and lung repair in a STAT6-dependent manner in both LPS and Pseudomonas aeruginosa bacterial pathogen-induced ALI (Acute Lung Injury) mouse models.
Keywords
Introduction
Macrophages are derived from the monocytic lineage precursor cells that are important for both the innate and adaptive immune responses. Different MΦ subtypes play a role in inflammation as well as in cancer. M1-MΦs produces high levels of proinflammatory cytokines, inducible nitric oxide synthase (iNOS), cyclooxygenase (COX)-2, and reactive nitrogen species [1] and thereby play a role in pro-inflammatory immunity. These M1-MΦs also take part in antitumor immunity by promoting and amplifying Th3-type responses, secreting TNFs, growth inhibitors and anti-angiogenic factors [2]. M2-type macrophages while possess anti-inflammatory activity, they can also develop protumor characteristics and promote tumor metastasis [3,4]. Tumor-associated MΦs (TAMs),or MΦs existing in the tumor microenvironment (TME) are mainly of M2-type which suppress antitumor immunity [5].
Here we will highlight the involvement of nanomaterials in the polarization of MΦs to its beneficiary form to fight against inflammation and cancer.
Macrophage Polarization
Macrophage Polarization chart in inflammation and cancer are shown as a schematic diagram in Figure 1.
MΦs Polarization in Inflammation: Inflammation in the lungs is one of the major concerns of health issues. M1 types of MΦs play a central role in the initiation and progression of ALI and/or ARDS [6-8]. Polarization of M1-MΦs to its M2 type and the later one secrete cytokines such as IL-4 or IL-10, IL-13, VEGFs, and TGFβ and initiate anti-inflammatory responses. Therefore, the effective control of active polarization of MΦs to its M2 type would help to control the lung injury, such as ALI/ ARDS.
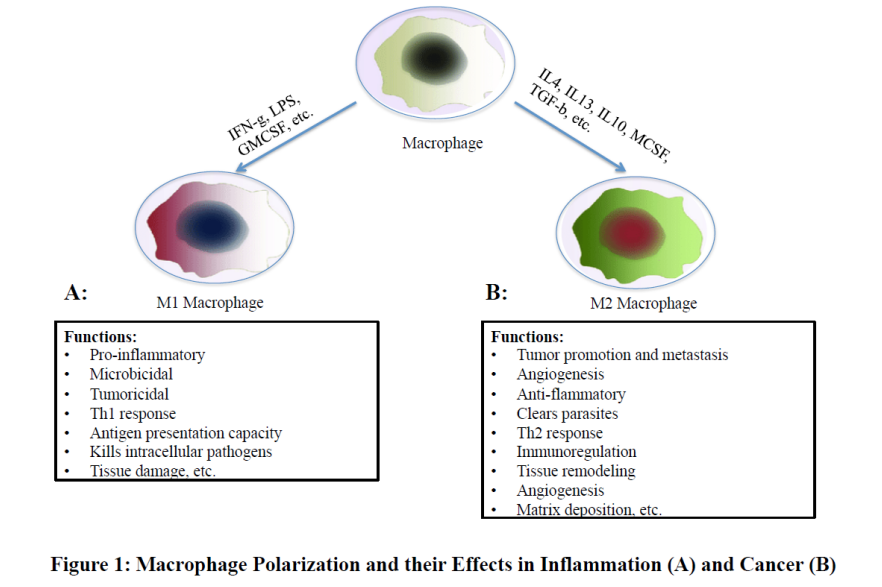
MΦs Polarization in Tumor Progression or Inhibition
Tumor associated MΦs (TAMs) are mostly M2-type. They secrete growth factors such as vascular endothelial growth factor A (VEGF-A) or placental growth factor (PGF) and increase new blood vessel formation by tumor endothelial cells [9]. TAMs also secrete matrix metalloproteinase-9 (MMP-9), serine proteases and cathepsins and degrade the extracellular matrix (ECM), facilitate tumor invasion and promote metastasis [10,11]. TAMs also enhance the chemo-resistance of cancer-associated stem cells, which allow tumor cells to regrow even after successful drug treatments [12]. Recent reports suggest that TAMs can suppress Natural Killer (NK) and T cell activity within tumors [13,14], which hinder targeted immune- as well as chemo-therapy of cancer cells. Since TAMs contribute to many interactions within tumors, TAMs are considered as a promising target cancer treatment [15].
Nanoparticles (NPs) and MΦs Polarization
NPs are very tiny particles (<100 nanometers) made up of latex, polymers, ceramic particles, metal particles, and carbon particles. Their surfaces could be hydrophilic or hydrophobic, exhibit surface charges and specific ligands, which are the reference factors for their selection to treat various clinical diseases [16]. Various synthetic NPs have been made using liposomes [17,18], polylactic-co-glycolic acid (PLGA) [19,20], chitosan [21], silica [22], dextran [23], and metals such as iron oxide or gold [24]. Applications of NPs in Medical science are increasing due to their physicochemical properties, e.g., size, shape, structure, chemical composition, morphology, and surface properties, etc.
Anti-Inflammatory Effect of NPs by Polarizing M1- to M2- MΦs (Figure 2)
NPs polarizes MΦs from an M1 to an M2 phenotype and thereby enables nanodrugs to enter the arthritic synovium and downregulates the pro-inflammatory cytokines, such as, IL-6, IL- 1β, and TNF-α and eventually stop the inflammation in arthritic rat models [25]. Gold nanoparticles (GNPs) were shown to inhibit the activation of NF-κB and interferon regulatory factors (IRFs), and therefore they facilitate the polarization of macrophages towards the anti-inflammatory M2 phenotype, and contribute to the reduction of lung inflammation and promotes the resolution phase during ALI [26].
Dextran-NPs becomes highly efficient and selective in targeting macrophages is due to the expression of dextran-binding C-type lectins and scavenger receptors on their surface [27]. Jain et al. already developed such a therapeutic regimen from those NPs to transport IL-10 into inflammatory environments to repolarize macrophages from an M1 to an M2 state, for the treatment of chronic inflammatory diseases [25].
Polyethylenimine NPs, carrying the gene for CD163 (an M2 macrophage marker) and grafted with a mannose ligand, can target M1 macrophages and convert it into M2 macrophages, leading to the rescue from inflammatory disease progression [28].
Anti-Cancer Effect of NPs by Polarizing M2 to M1-MΦs (Figure: 2)
With regard to TAMs targeting, two strategies can be adopted, one, depletion of TAMs, and second is the reprogramming of TAMs [29,30]. The first one can be achieved by blocking the recruitment of circulating inflammatory monocytes to the tumor site. Secondly, the inhibition of colony-stimulating factor 1 receptor (CSF1R) mediated signaling can cause the apoptosis of TAMs [31].

Further, inhibition of the CCL2–CCR2 signaling pathway, mononuclear cells can be arrested in the bone marrow resulting a decrease influx of MΦs in tumor [32-35]. Antibody against CCR2 and a small molecule CCR2 inhibitor (PF-04136309) were tried in a preclinical pancreatic cancer mouse model. These inhibitors resulted reduced recruitment of inflammatory monocytes to the tumors and reduced tumor growth and metastases [36,37]. Recent research shows that TAMs block acquired as well as innate immunity [38].
Now-a-days, many researchers have focused on small molecules and NPs formulations such as Toll-like receptor (TLR) agonists, cytokines, antibodies, and RNAs, for macrophage repolarization [39]. Polymeric NPs synthesized with an IL-12 cytokine to promote the conversion of M2 to M1-type, demonstrate the nanomaterials as a platform for cancer immunotherapy [40]. TLR agonists are potential agents to polarize TAMs into M1-like cells [41]. In 2018, Rodell et al. showed that R848, an agonist of TLR7 and TLR8, target TAMs and shift to an M1 phenotype, and eventually controls tumor growth [42,43].
Furthermore, mannose carbohydrate can also be employed to target macrophages. Immune cells express mannose receptors, therefore mannose become a popular ligand targeting macrophages [44,45]. Zhao et al. synthesized the albumin NPs having dual ligands, one, a transferrin receptor (TfR)-binding peptide T12, and the second is the mannose. They showed that this regimen efficiently can re-educate the protumor M2 to antitumor M1, and can inhibit the glioma cell proliferation successfully [46]. Most of mannose as a ligand to target macrophages are now being applied for macrophage re-polarization.
Interestingly, inhibition of CD40 also leads to IL-12 upregulation, which can repolarize TAMs into M1 macrophages. Similarly, inhibition of NF-κB signaling pathway can switch TAMs into M1 macrophages and block tumor cell’s growth. Therefore, this approach has a potential importance in cancer therapy [47].
However, when trying to target TAMs, the infiltration of nano drugs in the normal macrophages should be avoided. Further, to promote the retention of nano drugs in the tissues, a special methods like high interstitial fluid pressure (IFP) which contributes to NPs accumulation in tumors and restricts their extravasation and penetration, should be considered [48]. Another strategy is to make a pH-sensitive NPs. Since, the tumor pH ranges from 6.5 to 6.8 while the pH in healthy tissues is 7.4, NPs for instance, polymers, including poly(acryl amide) (PAAm), micelles and liposomes can release drugs through protonation or deprotonation designed to be pH-sensitive and bypass stably the normal tissues [16,49-53].
NPs Act as a Cargo of other Therapeutic Agents
NPs also can act as a vehicle of drug materials and overcome the limitations of several therapeutic agents, such as their non-specific distribution, potential toxicity, lack of targeting capability, poor solubility in water, stability in the system, and also to cross the Blood-brain barrier [16]. Chitosan molecules contain free amino acids, therefore can easily form salts in acidic solutions and also are cationic, making them useful as a antitumor drug carrier in an acidic TME followed by drug release [21]. Furthermore, nanomaterials can be enriched with different drugs together and/ or can be combined to carry different medications to overcome multidrug resistance challenges [16,54].


TheraCour BioPolymer Nanovehicles as an Anticancer Regimen
TheraCour platform polymer is a self-assembling, uniform, tailorable linear homopolymer that comprises polyethylene glycol (PEG) within its monomer unit which is heterochemically functionalized with a specially designed linker unit so that covalently connected aliphatic chains are suspended from it and separately site-targeting ligands are also covalently attached to it [55-57] (Figure 3).
This simple scheme results in a polymer that is like a half- biological membrane. In aqueous systems, it self-assembles into micelles with hydrophobic, flexible core region made of the lipid chains, hydrophilic ligands directing outwards into the aqueous milieu ready to seek their partners, connected together by the corona of PEG. Upon binding of the TheraCour polymeric micelle to a cellular receptor sought out by the ligand, multiple interactions with cellular receptors can take place resulting in increased avidity, due to the regular presentation of ligand at each monomer unit. This forces the corona close to the cell membrane and may initiate lipid mixing of the flexible pendant interior lipid chains of the micelle with the flexible lipids of the cell membrane, leading to passive fusion. Alternatively, receptor-mediated endocytosis can take place at properly chosen receptors. These processes would result in site-specified or address-targeted delivery of the encapsulated drug payload content of the micelle. As encapsulated rather than covalently immobilized, the drug payload can immediately go to work and does not have the latency of the need to be released from the polymer backbone in a covalent system.
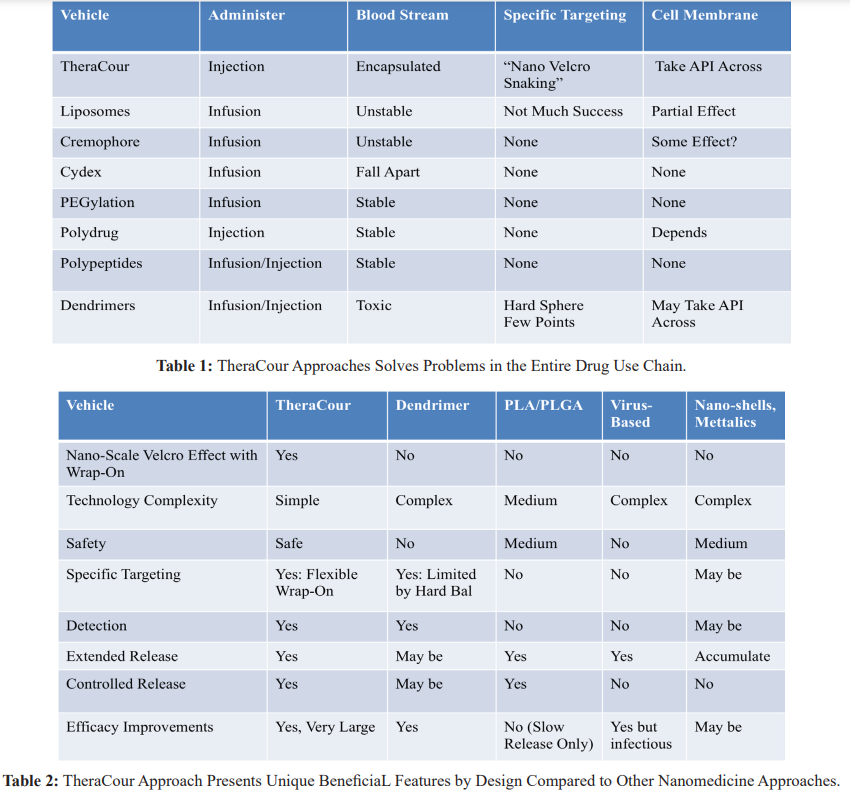
The TheraCour approache solves important problems in the entire drug use chain (Table 1), and has superior features by design to most other nanomedicine technologies (Table 2) [For Review, 58].
The graphical model of anticancer mechansim of TheraCour platform technogy are shown in Figure 4. [For Review, 58].
This polymer can effetively encapsulate many types of chemotherapeutic APIs, target the cancer cell based on the selected ligand, and thereby result in effective anticancer acitvity. Recently it was shown that the cell proliferation of two lung cancer cell lines (A549 and H441), and two breast cancer cell lines (SKBR3 and BT474) is mostly Inhibited by a folate-targeted TheraCour polymer delivering API (camptothecin, CPT) [58].
Discussion
Macrophages derives from monocytic precursor cells and play an important role in both innate and adaptive immune functions. They are also important linkers for adaptive immunity by presenting antigen and subsequently priming the T lymphocytes [59].
Further, the other significance of macrophages within the immune system is their heterogeneity and plasticity [60]. Depending on the exposure, factors like microbial stimuli (LPS) or cytokines (IL4, IL-10, IFNγ) macrophages could be polarized either to pro- inflammatory M1-type or to anti-inflammatory M2-type [61].
Functionally, these macrophage phenotypes are quite different than each other, especially in expressing the phagocytosis receptors such as CD16 and mannose receptor and also in their cytokine and chemokine production. Those make the difference in their ability to facilitate or suppress inflammation, scavenging debris and promoting tissue repair [62].
Therefore, a complete understanding of nanomaterials interaction with distinct polarized macrophage phenotypes, is important to translate the nanomedicines for clinical purposes. The major points to be considered are:
- Classically activated M1 macrophages are microbicidal and pro- inflammatory while alternatively activated M2 macrophages are predominantly immune modulators and anti-inflammatory [61].
- The differential uptake methods of nanoparticles by M1 and M2-type macrophages are complex, and involves cytoskeletal remodeling, membrane fusion and vesicular transport [63-65].
Recently it was reported that the polarization of macrophages towards the M1 phenotype resulted an increased uptake ability of non-PEGylated nanoparticles compared to their M2 counterparts [63]. In contrast, inhibition of CD47-SIRPα by anti-CD47 antibodies produced a higher pro-phagocytosis of cancer cells by M1-type as compared to M2-type macrophages [66]. However, how the M1/M2 polarization system works in the tissue-specific macrophages such as microglia in the central nervous system to cause neuro-inflammation remains unknown [67].
In brief, activated macrophage populations possess unique pro- and anti-inflammatory type that play an important role in immune regulation as well as in disease pathology. During strokes, for example, M1 macrophages becomes active and promote inflammation after ischemic injury, release cytotoxic cytokines and ultimately neuronal death [68]. In contrast, M2- like tumor associated macrophages promote immune suppression and facilitate tumor invasion [2]. Therefore, the understanding of the mechanism(s) how the nano-materials interact with specific macrophage phenotypes and the ability to design nano-materials for selective targeting to those macrophage subpopulations are crucial parts in designing the nano-medicines. here.
Conclusions and Future Perspectives
- Nanoparticle-based drug delivery systems offer a unique opportunity to target tumor-associated M2-macrophages, and inhibit progression of tumor growth and metastasis.
- In general, M2-type macrophages increase tumor growth and metastasis, suppress immune responses to cancer cells, while M1-type macrophages can selectively kill the cancer cells within the tumor microenvironment.
- However, standardized conditions are needed to measure the macrophage polarization by nanoparticles.
- Addition of macrophage-targeting moiety to the surface of a nanoparticle can enhance it’s macrophage polarization
- Further loading of the Macrophage-polarizing drug in the nanoparticles may exert a synergistic effects with their therapeutic ingredients.
- The literature suggests that polymeric nanoparticles and liposomes can cause M2-type polarization, while other types of nanoparticles can cause M1-type
- A variety of iron oxide nanoparticle treatments induce M1- type polarization in TAMs and reduce tumor growth in animal
Acknowledgement
We acknowledge all our colleagues and secretaries for their help during the preparation of the manuscript and providing all the relevant information. Thanks are due to Ms. Bethany Pond, a Chemist at AllExcel, Inc, for the English corrections.
Author contributions
Both of the authors contributed equally to preparing this article, reading and approving the final manuscript.
Conflict of interest
Both of the authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Gordon S, Taylor Monocyte and macrophage heterogeneity. Nat Rev. Immunol. 2005; 5: 953-964.
- Biswas SK, Mantovani Macrophage plasticity and interaction with lymphocyte subsets cancer as a paradigm. Nat Immunol. 2010; 11: 889-896.
- Murray PJ, Wynn Obstacles and opportunities for understanding macrophage polarization. J Leukocyte Biol. 2011; 89: 557-563.
- Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage Nat Rev Immunol. 2011; 11: 723-737.
- Mosser DM, Edwards Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008; 8: 958-969.
- Thompson BT, Chambers RC, Liu Acute respiratory distress syndrome. N Engl J Med. 2017; 377: 562-572.
- Huang X, Xiu H, Zhang S, et al. The role of macrophages in the pathogenesis of ALI/ARDS. Mediat 2018; 2018: 1264913.
- Lu HL, Huang XY, Luo YF, et Activation of M1 macrophages plays a critical role in the initiation of acute lung injury. Biosci Rep. 2018; 38: BSR20171555.
- Lin L, Chen YS, Yao YD, et CCL18 from tumor-associated macrophages promotes angiogenesis in breast cancer. Oncotarget. 2015; 6: 34758-34773.
- Chanmee T, Ontong P, Konno K, et Tumor- Associated Macrophages as Major Players in the Tumor Microenvironment. Cancers. 2014; 6: 1670.
- Afik R, Zigmond E, Vugman M, et Tumor macrophages are pivotal constructors of tumor collagenous matrix. J Exp Med. 2016; 213: 2315-2331.
- Sainz B Jr, Carron E, Vallespinos M, et Cancer Stem Cells and Macrophages Implications in Tumor Biology and Therapeutic Strategies. Mediators Inflamm. 2016; 2016: 9012369.
- Vitale M, Cantoni C, Pietra G, et al. Effect of tumor cells and tumor microenvironment on NK-cell Eur J Immunol. 2014; 44: 1582-1592.
- Movahedi K, Laoui D, Gysemans C, et al. Different Tumor Microenvironments Contain Functionally Distinct Subsets of Macrophages Derived from Ly6C(high) Monocytes. Cancer 2010; 70: 5728-5739.
- Hanahan D, Coussens Lisa Accessories to the Crime Functions of Cells Recruited to the Tumor Microenvironment. Cancer Cell. 2012; 21: 309-322.
- Sun T, Zhang YS, Pang B, al. Engineered nanoparticles for drug delivery in cancer therapy. Angew Chemie. 2014; 53: 12320-12364.
- Nguyen TX, Huang L, Gauthier M, et Recent advances in liposome surface modification for oral drug delivery. Nanomedicine. 2016; 11: 1169-1185.
- Ren H, He Y, Liang J, et al. Role of liposome size surface charge and PEGylation on rheumatoid arthritis targeting ACS Appl Mater Interfaces. 2019; 11: 20304-20315.
- Danhier F, Ansorena E, Silva JM, et PLGA-based nanoparticles an overview of biomedical applications. J Controlled Release. 2012; 161: 505-522.
- Acharya S, Sahoo PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv Drug Deliv Rev. 2011; 63: 170-183.
- Rao W, Wang H, Han J, et Chitosan- decorated doxorubicin-encapsulated nanoparticle targets and eliminates tumor reinitiating cancer stem-like cells. ACS Nano. 2015; 9: 5725-5740.
- Diab R, Canilho N, Pavel IA, et al. Silica-based systems for oral delivery of drugs macromolecules and cells. Adv Colloid Interface 2017; 249: 346-362.
- Ma L, Liu TW, Wallig MA, et Efficient targeting of adipose tissue macrophages in obesity with polysaccharide nanocarriers. ACS Nano. 2016; 10: 6952-6962.
- Mody VV, Siwale R, Singh A, et Introduction to metallic nanoparticles. J Pharmacy Bioall Sci. 2010; 2: 282-289.
- Jain S, Tran TH, Amiji M. Macrophage repolarization with targeted alginate nanoparticles containing IL-10 plasmid DNA for the treatment of experimental Biomaterials. 2015; 61: 162-177.
- Xiong Y, Gao W, Xia F, et al. Peptide-gold nanoparticle hybrids as promising anti-inflammatory nanotherapeutics for acute lung injury in vivo efficacy biodistribution and clearance. Adv Healthc 2018; 7: e1800510.
- Pustylnikov S, Sagar D, Jain P, et al. Targeting the C-type lectins-mediated host-pathogen interactions with dextran. J Pharm Pharm 2014; 17: 371-392.
- Alvarado-Vazquez PA, Bernal L, Paige CA, et Macrophage- specific nanotechnology- driven CD163 overexpression in human macrophages results in an M2 phenotype under inflammatory conditions. Immunobiology. 2017; 222: 900-912.
- DeNardo DG, Ruffell B. Macrophages as regulators of tumor immunity and immunotherapy. Nat Rev Immunol. 2019; 19: 369-382.
- Ostuni R, Kratochvill F, Murray PJ, et al. Macrophages and cancer from mechanisms to therapeutic implications. Trends 2015; 36: 229-239.
- Pyonteck SM, Akkari L, Schuhmacher AJ, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma Nat Med. 2013; 19: 1264-1272.
- Nywening TM, Wang-Gillam A, Sanford DE, et al. Targeting tumor-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer a single- center open-label dose- finding non-randomized phase 1b Lancet Oncol. 2016; 17: 651-662.
- Lim SY, Yuzhalin AE, Gordon-Weeks AN, et Targeting the CCL2- CCR2 signaling axis in cancer metastasis. Oncotarget. 2016; 7: 28697-28710.
- Zhang J, Patel L, Pienta KJ. CC chemokine ligand 2 CCL2 promotes prostate cancer tumorigenesis and Cytokine Growth Factor Rev. 2010; 21: 41-48.
- Qian BZ, Li J, Zhang H, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumor Nature. 2011; 475: 222-225.
- Kirk PS, Koreckij T, Nguyen HM, et al. Inhibition of CCL2 signaling in combination with docetaxel treatment has profound inhibitory effects on prostate cancer growth in Int J Mol Sci. 2013; 14: 10483-10496.
- Sanford DE, Belt BA, Panni RZ, et al. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer a role for targeting the CCL2/CCR2 axis. Clin Cancer Res. 2013; 19: 3404-3415.
- Gordon SR, Maute RL, Dulken BW, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumor Nature. 2017; 545: 495-499.
- van Dalen FJ, van Stevendaal M, Fennemann FL. Molecular repolarization of tumor-associated macrophages. Molecules. 2018; 24: 9.
- Wang Y, Lin YX, Qiao SL, et al. Polymeric nanoparticles promote macrophage reversal from M2 to M1 phenotypes in the tumor Biomaterials 2017; 112: 153-163.
- Shime H, Matsumoto M, Oshiumi H, et Toll-like receptor 3 signaling converts tumor-supporting myeloid cells to tumoricidal effectors. Proc Natl Acad Sci USA. 2012; 109: 2066-2071.
- Rodell CB, Arlauckas SP, Cuccarese MF, et TLR7/8-agonist-loaded nanoparticles promote the polarization of tumor-associated macrophages to enhance cancer immunotherapy. Nat Biomed Eng. 2018; 2: 578-588.
- Cully M. Cancer re-educating tumor-associated macrophages with Nat Rev Drug Disc. 2018; 17: 468.
- Irache JM, Salman HH, Gamazo C, et al. Mannose-targeted systems for the delivery of therapeutics. Expert Opin Drug 2008; 5: 703-724.
- Wang T, Zhang J, Hou T, et al. Selective targeting of tumor cells and tumor associated macrophages separately by twin- like core- shell nanoparticles for enhanced tumor-localized Nanoscale. 2019; 11: 13934-13946.
- Zhao P, Wang Y, Kang X, et al. Dual-targeting biomimetic delivery for anti-glioma activity via remodeling the tumor microenvironment and directing macrophage-mediated Chem Sci. 2018; 9: 2674-2689.
- Fong CH, Bebien M, Didierlaurent A, et An antiinflammatory role for IKK-β through the inhibition of classical macrophage activation. J Exp Med. 2008; 205: 1269-1276.
- Yang S, Gao Nanoparticles for modulating tumor microenvironment to improve drug delivery and tumor therapy. Pharmacol Res. 2017; 126: 97-108.
- Fukumura D, Jain Tumor microenvironment abnormalities causes consequences and strategies to normalize. J Cell Biochem. 2007; 101: 937-949.
- Kost J, Langer R. Responsive polymeric delivery systems. Adv Drug Deliv 2001; 46: 125-148.
- Kim JO, Kabanov AV, Bronich TK. Polymer micelles with cross-linked polyanion core for delivery of a cationic drug J Control Release. 2009; 138: 197-204.
- Lo CL, Huang CK, Lin KM, et Mixed micelles formed from graft and diblock copolymers for application in intracellular drug delivery. Biomaterials. 2007; 28: 1225-1235.
- Borchert U, Lipprandt U, Bilang M, et al. pH-induced release from P2VP-PEO block copolymer vesicles. Langmuir. 2006; 22: 5843-5847.
- Bao G, Mitragotri S, Tong S. Multifunctional nanoparticles for drug delivery and molecular imaging. Ann Rev Biomed 2013; 15: 253-282.
- https://www.bloomberg.com/press-releases/2021-03-09/ nanoviricides-inc-pan-coronavirus-covid-19-drug-candidates- are-highly-effective-in-pre-clinical-animal-studies-i.
- Barton RW, Tatake JG, Diwan AR. Nanoviricides- A Novel Approach to Antiviral Bionanotechnology II.Ed. David E. Reisner. CRC Press. Taylor and Francis GroupnBoca Raton FL. 2011; 141-154.
- Barton RW, Tatake JG, Diwan AR. Nanoviricides Targeted Anti-Viral Nanomaterials Handbook of Clinical Nanomedicine Nanoparticles Imaging Therapy and Clinical Applications. Raj Bawa Gerald F. Audette, Israel Rubinstein. ISBN 9789814669207. Published February 24 by Jenny Stanford Publishing. 2016; 1039-1046.
- Anil Diwan, Jayant Tatake, Ashok Chakraborty. Therapeutic Uses of TheraCour™ Polymeric Nanomicelles Against Cancer Infectious Diseases and More. Book: Nanomaterials for Cancer Detection Using Imaging Techniques and Their Clinical Springer Nature. 2022.
- Iwasaki A, Medzhitov R. Regulation of Adaptive Immunity by the Innate Immune Science. 2010; 327: 291-295.
- Sica A, Mantovani Macrophage plasticity and polarization in vivo veritas. J Clin Invest. 2012; 122: 787-795.
- Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005; 23: 344-346.
- Mantovani A, Sica A, Sozzani S, al. The chemokine system in diverse forms of macrophage activation and polarization. Trends in Immunol. 2004; 25: 677-686.
- Walkey CD, Olsen JB, Guo H, et al. Nanoparticle Size and Surface Chemistry Determine Serum Protein Adsorption and Macrophage Uptake. Journal of the American Chemical 2012; 134: 2139-2147.
- Moreno JL, Mikhailenko I, Tondravi MM, et IL-4 promotes the formation of multinucleated giant cells from macrophage precursors by a STAT6-dependent homotypic mechanism contribution of E-cadherin. J Leukoc Biol. 2007; 82: 1542- 1553.
- Montaner LJ, da Silva RP, Sun J, et al. Type 1 and type 2 cytokine regulation of macrophage endocytosis differential activation by IL-4/IL-13 as opposed to IFN-γ or IL-10. J 1999; 162: 4606-4613.
- Zhang M, Hutter G, Kahn SA, et al. Anti-CD47 Treatment Stimulates Phagocytosis of Glioblastoma by M1 and M2 Polarized Macrophages and Promotes M1 Polarized Macrophages PLoS ONE. 2016; 11: e0153550.
- Prinz M, Priller J. Microglia and brain macrophages in the molecular age from origin to neuropsychiatric disease. Nat Rev 2014; 15: 300-312.
- Hu X, Li P, Guo Y, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012; 43: 3063-3070.