Therapeutic Apheresis, Immunosuppression and Human Monoclonal Antibodies in Nephrology
Author'(s): Rolf Bambauer1*, Ralf Schiel2, Octavio J. Salgado3 and Richard Straube4
1University of Saarland, formerly: Institute for Blood Purification, 66424 Homburg/Saar, Germany
2Clinic for Metabolic Diseases, Medigreif Inselklinik, 127424 Heringsdorf, Germany.
3School of Medicine, Universidad Católica de Cuenca, Ecuador
4INUSpherese® INUS Medical Center, Tagesklinikum, 93413 Cham, Germany.
*Correspondence:
Rolf Bambauer, MD, PhD, Frankenstrasse 4, 66424 Homburg/ Saar, Germany, Tel: 0049(0)68412-68500, Fax: 0049(0)6841- 68561.
Received: 21 Sep 2023; Accepted: 03 Nov 2023; Published: 09 Nov 2023
Citation: Bambauer R, Schiel R, Salgado OJ, Straube R. Therapeutic Apheresis, Immunosuprresion and Human Monoclonal Antibodies in Nephrology. Clin Immunol Res. 2023; 7(1): 1-16.
Abstract
Most of the immunologic or non-immunologic renal diseases have a bad prognosis without treatment, and the treatment is complicated in these diseases. With the introduction of therapeutic apheresis, since more than 45 years, has led in combination with immunosuppressive therapies and/or human monoclonal antibodies to a steady increase in survival rates over the last years. Therapeutic apheresis is accepted as supportive therapy in all severe renal diseases such as acute kidney injury, nephrogenic systemic fibrosis, rapid progressive glomerulonephritis, hemolytic uremic hemolytic syndrome, and in renal transplant rejection. Other modern therapy concepts are different human monoclonal antibodies with or without therapeutic apheresis. For these renal disorders, besides the pathological aspect, the first-line and second-line therapies are shown. Therapeutic apheresis has been shown to effectively remove antibody, toxic substances and others from blood and lead to rapid improvement. The guidelines of the Apheresis Applications Committee of the American Society for Apheresis are cited.
Keywords
Abbreviations
AAC: Application Committee, AAV: ANCA associated vasculitis; ab: Antibody, ABM-ab-GN: Anti-basement Membrane Antibody Glomerulonephritis, ABO: Blood group system, ADAMTS13: A Disintegrin and Metalloproteinase with a Thrombospondin type 1 motif Member13. AID: Autoimmune Diseases, AKI: Acute Kidney Injury, ALG: Anti-Lymphocyte Globulin, AMR: Antibody-Mediated Rejection, ANCA: Antineutrophil Cytoplasma Antibodies, ASFA: American Society for Apheresis, auto-ab: autoantibody, ATG: Anti-Thymocyte Globulin, BW: Body Weight, CIC: Circulating immune complex, CKD: chronic kidney disease, Cr: creatinine, DAH: diffuse alveolar hemorrhage, DFPP: Double Filtration Plasmapheresis, DIC: Disseminated intravascular coagulation, DSA: donor-specific antibody. ECP: extracorporeal photopheresis, EHEC: enterohemorrhagic E. coli, ESRD: end stage renal disease, Fc: fragment crystallizable, FFP: fresh frozen plasma, FSGN: focal sclerosing glomerulonephritis, FSGS: focal sclerosing glomerulosclerosis, GBM: glomerular basement membrane, Gd: gadolinium, GFR: glomerular filtration rate, GN: glomerulonephritis, HD: Hemodialysis, HLA: Human Leukocyte Antigen, HMA: Human Monoclonal Antibodies, HUS: Hemolytic-Uremic Syndrome, HVP: High Volume Plasma Exchange, IA: Immunoadsorption, IC: Immune Complex, ICN: Immune Complex Nephritis, IS: Immunosuppression, IVIG: Intravenous Immunoglobulin, LA. Lipid Apheresis, MCGN: Minimal Change Glomerulonephritis, MCP: Membrane Cofactor Protein, MEPEX: Plasma Exchange for Renal Vasculitis Study, MMF: Mycophenolate Mofetil, mTOR: Mechanistic target of Rapamycin, MPGN: Membranoproliferative Glomerulonephritis, MW: Molecular weight, NS: Nephrotic Syndrome, NSF: Nephrogenic Systemic Fibrosis, NS: Nephrotic Syndrome, PRA: Panel-Reactive Antibody, RES: Reticuloendothelial System, TA: Therapeutic Apheresis, TAC: Tacrolimus, TBV: Total Blood Volume, TMA: Thrombotic Microangiopathy, TPE: Therapeutic Plasma Exchange, TPV: Total Plasma Volume.
Introduction
Severe diseases with immunologic or non-immunologic origin were treatable with the introduction of hollow fiber modules as therapeutic apheresis since more than 45 years ago. The advantage of these hollow fiber modules over centrifuges is a complete separation of the cellular components from plasma and due to increased blood flow rate higher efficacy. However, cell damage such as thrombocytes occurs less using membranes than centrifuges for extracorporeal blood separation methods [1]. Enormous numbers of technological, economical and social factors have an impact on the practice of apheresis [2]. The modern adsorption technologies with special developed columns allow a better selective separation of the plasma toxins, antibodies and other pathologic substances without the use of any substitution solutions.
Hafer et al. shown using centrifuges, therapeutic apheresis (TA) has shorter treatment time such as using hollow fiber membranes [3]. This is no advantage, more important is to keep the blood levels with antibodies, and/or other pathogenic substances on a very low level over long time during treatment. In that time, the substances that should be eliminated could invade into the intravascular space and eliminated than by the membrane separators.
In Nephrology, mostly used therapeutic plasma exchange (TPE) using hollow fiber modules and adsorption technologies with special columns, as many of these membranes can be used with currently available dialysis equipment [4]. The nephrologists have an extensive training in the management of blood purification treatments including vascular access, anticoagulation, volume management and prescription for solute clearance. Indications in nephrology for TA expand the clinical practice of nephrologists [5]. Especially, renal diseases with an immunologic origin and available antibodies in the blood are indications for TA, but also non-immunologic renal diseases could be indicated for TA [6,7]. Therapeutic apheresis is the generic term for all extracorporeal blood purification methods to remove antibodies and other toxic and pathogenic substances from blood. There are only a few prospective controlled trials available which are of adequate statistical power to definitive conclusions to be reached regarding the therapeutic value of TPE. This means, many diseases are rare and under investigation. Many investigators have to compensate this grouped heterogenous diseases together, often retrospectively, and used historical controls. The latter design is potentially hazardous, given that earlier diagnosis, recognition of milder cases, and improved general care over time may be lost as a benefit of TA [7]. The details of treatment protocols vary widely between centers, rendering it difficult to compare studies.
In inflammatory renal diseases TA is primarily used as an adjunct to conventional immunosuppressive therapy and might be expected a priori to confer only small additional benefit that require large sample size for its detection. Most renal disorders with immunological origin requires TA, immunosuppression (IS) with steroids, cytotoxic agents, metabolites and human monoclonal antibodies (HMA). The total therapy is individually tailored to the needs of the patient [8,9].
Therapeutic apheresis methods, which are used in nephrology, are TPE, double filtration plasmapheresis (DFPP), high volume plasma exchange (HVP), immunoadsorption (IA), adsorptive cytapheresis, and extracorporeal photopheresis (ECP) [1,10]. For the diseases for which TA is used, the Apheresis Application Committee (AAC) of the American Society For Apheresis (ASFA) are cited [10,11]. The authors try to give a review of the most pathogenic aspects indicting that TA, IS and/or HMAs could be a supportive therapy in nephrology.
Nephrological Diseases
Most diseases treated with TA are immunologic diseases. The etiologies of most of these diseases are unknown. Viral infections and other influences can lead to altered native antigen with a loss suppression [8]. Antinuclear antibodies are to be found against most nuclear structures. Vasculitis is found in all these diseases, and is most easily demonstrated histological in the precapillary arterioles and post-capillary venuoles [12]. In autoimmune diseases (AID) the autoimmunization is responsible for the disease condition, in those in which it possibly has a major influence on the further course of the diseases, and those in which the auto-immunization phenomena are probably only for diagnostic importance [12].
The release of much different soluble substances as antibodies (ab) that diffuse away from the site of their production accompanied by a complex set of events what is called inflammation. Autoantibodies are not necessarily auto-aggressive or destructive. They only lead to inflammatory tissue reaction when, through their binding to cells and through complement activation, the reaction chain of the serum complement system is triggered [12]. Immune complex (IC) is a physiological process and serves to eliminate foreign material, such as bacteria, the components and viruses. In the liver and the spleen, the formed ICs are removed from blood by the adhesion of the Fc-Fragments of the abs to the corresponding phagocyte receptors. Immune complexes are deposited preferentially in certain sites such as the kidneys, joints, lungs and skin. In the kidneys accumulate ICs because the blood pressure in the glomerular capillaries is four times higher than in other capillaries and because the glomerulus retains ICs by a simple filtering effect [12]. Similarly, ICs may also accumulate in other body filters. Circulating ICs (CIC) are involved in the regulation of various immune phenomena. It is possible to interrupt the pathological process by eliminating ab with TA.
Acute Kidney Injury (AKI)
Acute kidney injury is a life threatening and organ threatening therapeutic challenged that require prompt, accurate diagnosis and treatment and remains reversible damage with oliguria or anuria [13]. Acute kidney injury is defined as acute, over hours or days, developing renal function damage, which is measured with the glomerular rate (GFR), and in some cases can show a polyuric course. The damage of the kidney varies depending on the degree and duration of pre-renal, renal or post-renal noxae [14,15].
The classification of the factors in pre-renal, renal, and postrenal disorders offers new therapeutic possibilities [6]. The pre-renal disorders are circulatory-ischemic disorders such as reduced blood pressure or volume, which can be directly influenced therapeutically. Exogenous, endogenous toxins, other pathogenic substances, and especially sepsis by Gram-negative cocci, as renal factors, can easily lead to severe renal injury. In these cases, primarily are the application of antibiotics combined with intravenous immunoglobulin G (IVIG), and in severe cases, TA together with hemodialysis (HD) could begin so early as possible to eliminate metabolic products [16]. Here, early implementation of TA interrupts pathogenic chain reactions during the damage phase, and a recovery is accomplished through the elimination of toxins and pathogenic substances. The exogenous toxins can lead to AKI through tubular necrosis are mercurous chloride (sublimate), carbon tetrachloride. Glycol and oxalic acid cause extensive cortical necrosis, usually with irreversible renal damage. Heavy metals, together with organic solvents, foreign proteins, substances with high molecular weight, and/or strong binding are further nephrotoxins [6]. In COVID 19 with available abs and AKI, TA and HD is also indicated in an early stage [17].
Triggering factors such as the endogenous toxins like free hemoglobin and myoglobin can be transfusion reactions, which result in hemolysis after HD, severe operations and disseminated intravascular coagulation (DIC), burns, tissue destruction and hemolytic uremic syndrome, which can be quickly eliminated by TA [6]. A part of the endogenous toxic renal damage is the cast nephropathy of plasmocytoma. The most common renal manifestations associated with cancer include AKI in the setting of multiple myeloma, tumor lysis syndrome, post-hematopoietic stem cell therapy, and AKI associated with chemotherapy [18]. Here, TA and HD are also indicated in an early phase of the disease to improve the outcome.
Acute kidney injury can occur due to diseases of the liver, as hepato-renal syndrome, decomposition products, toxic and phenols are released through the destruction of liver cells due to liver insufficiency. A reduced effective blood volume, and the increased presence of vasoactive, humoral, or neuro-humoral substances, occurring as a result of liver damage, leading to vasoconstriction of the kidney and thus insufficient blood flow are the pathogenesis of hepato-renal syndrome [6]. Acute pancreatitis can be caused by DIC aggravated by dehydration and often associated with AKI. Responsible for renal damage are toxic digestive enzymes released into the systemic circulation by the diseased pancreas. Active enzymes released by autolysis are amylases, esterases, nucleases, and inactive proenzymes, such as proteinase, peptidases and phospholipase A, which is activated by trypsin, releases highly toxic lysolecithin from lecithin, which can damage the organs in particular the kidney leading to AKI [19]. In severe cases, TA should be considered to eliminate the toxic substances and thus revert or reduce damage, prior to surgical intervention. The almost invariable fatal outcome of acute hemorrhage necrotizing pancreatitis can be prevented in some cases through the early introduction of TA.
There are not been enough controlled studies of TA in the treatment of AKI. It is difficult to make larger controlled studies, therefore, the physician who treats patients with AKI must decide from themselves whether the introduction of TA in AKI is indicated or not. Acute kidney injury is a life threatening disease. In this point of view, it is useful to discuss the disease risk factors and therapeutic possibilities with all persons involved in such case.
Nephrogenic Systemic Fibrosis (NSF)
Nephrogenic systemic fibrosis is a rare but severe systemic disease in patients with acute or chronic kidney disease (CKD), and often associated with the administration of gadolinium (Gd) [20]. It occurs in 3-7% of patients with renal insufficiency receiving Gd, however, in patients with a GFR < 30 ml/min/1.73 m², in current thrombotic or inflammation, and in higher dose of Gd, in patients with hepato-renal syndrome and in perioperative period following liver transplantation [21]. Other causal factors include surgery, systemic infections, metabolic acidosis, high erythropoietin blood levels, and elevations of calcium, iron, copper, and phosphate.
Nephrogenic systemic fibrosis is onset 2-4 weeks after Gd administration. However, the range can be from 2 days to 8 years, than the skin shows a symmetrical erythematosus rash, non-pitting edema, paresthesia, and pruritus in the extremities. Other symptoms can be hair loss, gastroenteritis, conjunctivitis, bilateral pulmonary infiltrates and fever. These symptoms results in joint contractures leading to wheel-chair dependence and may into deeper tissue including skeletal, heart, pericardium, pleura lungs diaphragma, esophagus, kidneys, and testes [11]. An overall mortality rate is up to 30%. The disorder progresses to death with weeks to months, in a small group of patients with recovered renal function, the disease can enter in remission [10]. The etiology is most unknown. Gadolinium may also directly fibrosis, and the administration must avoid in patients with GFR < 30 ml/min/1.73 m2.
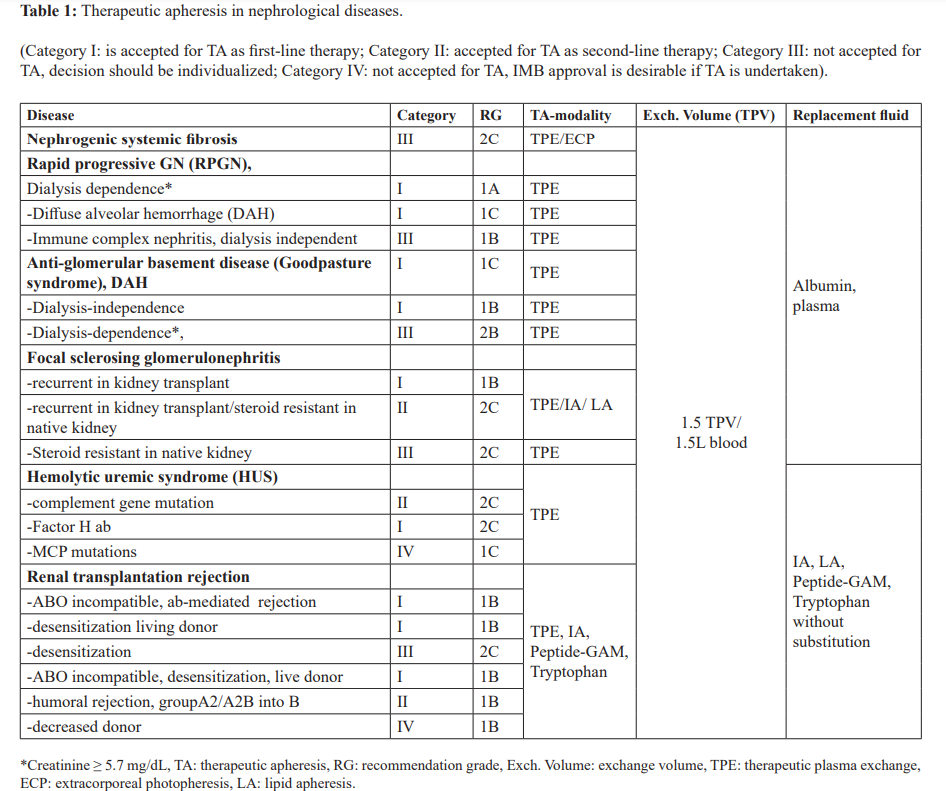
One to three HD sessions can remove 97 to 99% of the dose of GD. Other therapies include IVIG, alefacept, pentoxifylline, imatinib, messy late, chelation therapy with sodium thiosulfate, TPE, or ECP, or renal transplantation [22-26]. With TPE or ECP improvement of skin, increased range of motion improved ambulation and from wheel-chair bound to walking, decreases swelling, pain and paresthesia are reached. Therapeutic plasma exchange with a 1.5 total plasma volume (TPV) is daily introduced and in total 10-14 treatments [10]. The ECP of 1.5 L of whole blood volume various to 2 total volume (TBV). The frequency is from 2 in consecutive days every 2-4 weeks up to 5 sessions every other day, and the duration of ECP could be 4-16 months [25]. The AAC of the ASFA has given the NSF the category II and the recommendation grade (RG) 2C (Table 1) [10,11].
Rapid Progressive Glomerulonephritis (RPGN)
Rapidprogressiveglomerulonephritisisadiffuseglomerulonephritis (GN) which frequently starts acutely. As a histologic diagnosis, RPGN can occur from a number of etiologies, including a rare anti-basement membrane antibody glomerulonephritis (ABM- ab-GN), antineutrophil cytoplasma antibodies (ANCA), and IgA nephritis. Emboli with necrosis of the capillary walls and semi-lunar formation, and deposition of IgG and C3 along the glomerular basement membrane are usually the histological characteristics. Most patients are simultaneously accompanied by AKI [27]. In their circulation, more than 90% of patients with RPGN due to Goodpasture´s/anti-glomerular basement membrane (GBM) RPGN have anti-GBM abs.
A rapid loss of renal function with the histological findings of crescent formation in over 50% of glomeruli is found in RPGN [27]. A proliferation of cells within Bowman´s space of the glomeruli due to the extravasations of proteins into the space is found histologically. These cells consist of proliferating parietal epithelial cells as well as infiltrating macrophages and monocytes. Rapid progressive glomerulonephritis is a clinical syndrome with a different etiologies. Immunoglobulin G, as linear disposition, due to autoantibodies (auto-abs) to type IV collagen representing antiglomerular basement GN in 15% [28].
The incidence is 0.85 per 100.000/year [29]. The first therapy includes high-dose corticosteroid (e.g., methylprednisolone), and cytotoxic immunosuppressive drugs (e.g., cyclophosphamide, azathioprine) [10]. Further drugs are leflunomide, deoxyspergualin, tumor necrosis factor blockers, calcineurin inhibitors, antibodies against T cells or rituximab [28,30]. In antineutrophil cytoplasmic antibody-associated vasculitis, an oral C5a receptor antagonist,avacopan, was approved as an adjunctive therapy. Patients treated with avacopan had more rapid reduction in albumuria and greater recovery in GFR [31].
The rationale for TA is the RPGN with dialysis dependence (Creatinine > 6 mg/dL) and the RPGN with diffuse alveolar hemorrhage (DAH). Both has received the Category I and RG 1A and 1C from the AAC of the ASFA, and the RPGN dialysis independent has the Category III with the RG 2C (Table 1) [10,11]. Therapeutic apheresis was applied to all causes of RPGN, because of the benefit of TPE [11,32]. In some trials, the role of TPE has been investigated in pauci-immune and immune complex GNs. Results indicated that TPE may be beneficial for dialysis dependent patients with severe renal dysfunction, however, is no therapeutic benefit over immunosuppression in milder disease. In these trials, the predominance of pauci-immune GN cases may account for these results [11].
Immunoadsorption removes effectively pathogenic immune complexes from the blood [33]. The frequency of TA is every other day, and the volume treated is 1-1.5 TPV, the substitution solution could be a human-albumin 5% electrolyte solution in TPE. The duration of TA therapy is not well defined but usually 1-2 weeks followed by tapering with less treatments. In some trials, TA was stopped, because there was no response after 4 weeks of treatment. In the international controlled study, PEXIVAS, TPE was compared versus no TPE and standard versus reduced dose steroid regimen on the primary composite outcome of end-stage renal disease (ESRD) or death in patients with ANCA-associated vasculitis (AAV), which is the largest study on the role of TPE in AAV [34]. Therapeutic plasma exchange was not significantly associated in patients with their risk of primary outcomes, mortality, and other side effects, therefore. Hence, it was suggested that TPE might be effective in suppressing ESRD in the early stage of treatment [35]. PEXIVAS study did not show the addition of TPE to standard therapy conferred benefits in patients with severe AAV, however, it did show that a reduced-dose regimen of oral glucocorticoids was non-inferior to a standard-dose regimen [36,37].
Immune Complex Nephritis (ICN)
The deposition of immune complexes (ICs) can cause many types of glomerulonephritis, which induce tissue injury via either engagement of Fc-receptors on effector cells or via complement activation [38]. The consequence of systemic autoimmune disease is to trigger by the generation of antibody and the subsequent tissue deposition of ICs. A modulation of the autoantibody response disrupts pathogenesis by preventing the formation of ICs. Uncoupling IC formation from subsequent inflammatory response seems unlikely because of the apparent complexity of the IC- triggered inflammatory cascade [39].
The therapeutic strategies are not as clear in idiopathic symptomatic RPGN as in anti-glomerular basement membrane antibody nephritis. If pulse therapy (high dose therapy with corticosteroids) or TPE, an improvement in renal function is possible in more than 60% of the patients. Therapeutic plasma exchange in combination with immunosuppression should be carried out as quickly as possible, in view of the devastating pathophysiologic consequences of interaction between CIC and the basement membrane [40].
A combination of corticosteroid and cyclophosphamide or rituximab or eculizumab, and/or mycophenolate mofetil (MMF), and tacrolimus (TAC) is indicated as therapy for remission induction of ANCA-associated vasculitis [40-42]. This multi- target therapy of corticosteroid or HMA, MMF and TAC has shown a remission-induction of intractable ANCA-associated glomerulonephritis developed independently of systemic lupus erythematosus.
Anti-basement Antibody Glomerulonephritis (Goodpasture Syndrome, ABM-ab-GN)
Anti-basement membrane antibody glomerulonephritis is a rare life-threatening small-vessel vasculitis affecting pulmonary capillaries, glomerular capillaries or both which is caused by the deposition in alveolar and glomerular basement membrane of circulating autoantibodies [38]. The incidence is 0.5-1 per million population [39]. Patients commonly represent a rapidly progressive glomerulonephritis, accompanied by alveolar capillaries and pulmonary hemorrhage. Antibodies, in ABM-ab-GN, are directed against a peptide component of one of the two non-collagen parts of type IV collagen. Type IV collagen is found not only in the kidney, but also in the vessels of the lung [43]. The autoantibodies in the basement membrane of the glomeruli in the kidney and alveolar capillary walls in the lung, leading to severe organ injury with a high risk of morbidity and mortality [44]. The mechanisms for the production of autoantibodies against the antigens are not clarified.
With Goodpasture syndrome, a large number of diseases have been associated based on different cases. The most reported associations are with membranous nephropathy and antineutrophil cytoplasmatic- associated vasculitis. Only a small part of ANCA GN have anti GBM ab, mostly it seems to be an environmental or infectious exposure that triggers these diseases. It has been speculated that both membranous and ANCA-positive vasculitis damage to the kidney elicits an immune response against the GBM, leading to the production of autoantibodies [45].
The production of anti-basement abs is frequently limited in duration. The auto-abs cause severe disturbances in the permeability in the lung with severe deterioration in diffusion capacity and hemoptysis. The deposition of these auto-abs in the kidney leads to rapid deterioration in renal function. Histologically, it expresses in a necrotizing GN in part. The linear deposits of IgG can be immune-histologically seen at the basement membranes of the lungs and the kidney [35,36]. An antigen with a probable size of 26,000 – 28,000 Daltons is considered responsible for this deposits, its immunogenic epitopes being located on the stable glomerular domain NC1 of collagen IV [38]. In a hexamers form and forms monomers and dimers, the antigen is presented [36,38]. Antigen determinants are exposed after dissociation and can thus bind specific abs. In all basal membranes, in particular in those of the glomeruli, renal tubuli, the Bowmann capsule, the lung, and the plexus chorioideus, in the placenta, and also in those of the aorta and the small intestine, these molecules seems to be present.
After De Lind van Wijngaarden et al., acute and chronic tubulointerstitial lesions predict the GFR at 12 months, it was the use of TPE and the number of normal glomeruli on biopsy that remained positive predictors of dialysis independence in the same time interval [46]. Unaffected glomeruli determine long- term renal outcome at 1 year. The same group extended their work in determining the rate of renal recovery [47]. The plasma exchange for renal vasculitis study (MEPEX) showed in 69 dialysis dependent patients who were part of the TPE trial, that TPE was superior to pulse corticosteroid therapy with respect to the change of coming of dialysis. The outcome was measured of normal glomeruli.
Many patients with an ABM-ab-GN become dialysis dependent, therefore an aggressive therapy with daily TPE, pulse therapy of intravenous corticosteroids, and cytotoxic drugs [48,49]. In few reports the successful treatment is reported with MMF, and cyclosporine [38]. In several case reports, rituximab, an HMA, and imliflidase were successful as either “add on” to standard therapy. Rituximab showed a rapid reduction of anti-GBM abs but without benefits on the renal outcome [38]. With therapeutic apheresis the pulmonary bleeding can stopped and improved in cases, which based on the same immunological process, even when renal function is already irreversible impaired [49]. A final long- term prognosis for patients whose condition improved after TA cannot be made. Recovery, or at least partial recovery is possible, as basement membrane antibody formation often ceases during treatment. Even in patients with ABM-ab-GN after COVID-19 mRNA vaccination, the treatment with pulse methylprednisolone, cyclophosphamide and TA was successful [50].
The AAC of the ASFA has given the ABM-ab-GN, with dialysis independence and/or DAH, the Category I and the RG 1C and 1B, respectively, and with dialysis dependence and no DAH the Category III and the RG 2B (Table 1) [10,11]. The frequency of TPE or IA is every or every other day until anti-glomerular basement membrane antibodies are detectable. The treated volume is 1-1.5 TPV, and the substitution solution in TPE could be a 5% human albumin-electrolyte solution and/or fresh frozen Plasma (FFP). The treatment is for 1-2 week followed by tapering with less frequent treatments. The duration of therapy is not well defined in the literature, and TA should be carried out until abs fall to undetectable levels in patients with active disease and abs [11].
RPGN with or without Glomerular Deposition (ANCA-ab), Pauci-Immune RPGN
About 60% of patients with RPGN present with crescentic glomerulonephritis characterized by few or absent immune deposits, the so-called pauci-immune RPGN. These patients have either Wegener´s granulomatosis, ANCA-ab associated vasculitis, polyarthritis nodosa, or “renal-limited” pauci-immune GN [51]. A single disease may represent a spectrum of manifestations, because there is marked overlap of clinical and histopathologic features, and many patients have ANCAs in their blood which are more common that anti-GBM. The ANCAs correlate in some patients with the disease activity, and the ANCAs may contribute to the pathophysiology of pauci-immune RPGN through reactivity with neutrophils or endothelial cells, and/or other inflammatory mechanism [52,53].
The pauci-immune RPGN has in general a poor prognosis. It is difficult to get an exact therapeutic recommendations from the literature, due to the different types of RPGN in the investigated trials. About 80% of such patients progress to ESRD without therapy with pulse corticosteroids, high dose immunosuppression or cytotoxic drugs. In some trials, TA as an adjunct to conventional therapy in patients with pauci-immune RPGN, have been evaluated of benefit [54,55]. In milder forms of pauci-immune RPGN, the results of randomized trials argue against the role for TA. However, in patients with severe disease, when TA is used as adjunct to immunosuppressive therapy, it suggest a potential benefit. This relative lack of efficacy reflects the efficiency of immunosuppressive agents in halting inflammation and preserving renal function in most patients. This is supported by the results of uncontrolled studies, suggesting a response rate of 70% in patients with RPGN treated with TA, similar to that of patients treated with immunosuppressive therapy with a response rate of 60%. Therapeutic apheresis should be introduced in the early phase of the disease as possible.
De Lind van Wijngaarden et al. have shown in the MEPEX study, that in patients with dialysis-dependence, ANCA-associated vasculitis, the chances of recovery differ depending on the type of adjunctive treatment, the percentage of normal glomeruli and glomerulosclerosis, the extend of tubular atrophy, and the presence of arteriosclerosis. Even with an ominous biopsy at diagnosis in combination – with dialysis dependence, the chance of renal recovery exceeds the chance of therapy-related death when TPE is used as adjunctive therapy [47]. However, the PEXIVAS study did not show that the adjunctive TPE to conventional therapy conferred benefits in patients with severe ANCA-associated vasculitis, but it showed a reduced-dose regimen of oral glucocorticoids was non- inferior to standard-dose regimen [46]. In patients with high titers of circulating ICs or other antibodies, which could damage the kidney and other organs, IA with protein-A can be more effective than TPE [56].
Recommendations for Therapy of RPGN
The therapy possibilities were extended in last 45 years to include TA. With TPE, antigens, antigen-antibody complexes, and ICs can be eliminated from the blood. Immunomodulation through suppression or stimulating of antibody formation, as well as temporary remission of the inflammation through inhibition of the mediators, will be enabled by a corresponding therapy. The combination of TA and immunosuppression therapy seems to be advisable, particularly in view of the unfavorable prognosis for RPGN, with its complex causes.
The recommendations are based on a few uncontrolled and controlled studies available [57-62]. In combination with the immunosuppression with prednisolone (intravenous pulse or oral therapy), cyclophosphamide (intravenous pulse or oral therapy), or azathioprine, and/or rituximab, TA is indicated in the following cases:
- RPGN with serum creatinine ≤ 8.mg/dL without oliguria in anti-GBM disease.
- All severe forms of RPGN with or without ANCA ab, like the pauci-immune complexes (Cr ≥ 6 mg/dL or patient on dialysis).
- Goodpasture syndrome with life-threatening hemoptysis, or diffuse alveolar hemorrhage from ANCA or MPA independent of renal function status [28].
Even in elderly patients, TA is used for renal diseases, and is relatively safe. Trends towards death in these group of patients may be multifactorial and not necessarily related to TA [63]. With TA a decrease end of end-stage renal disease or death in patients with RPGN [64]. Different authors recommend a combination of TA with immunosuppressive therapies including biologics, which seems to be more effective than TA alone [28,65,66]. Others prefer in cyclophosphamide-resistant ANCA-associated GN a multi- target therapy, a combination of corticosteroids, MMF, and TAC [41]. However, for both additional investigations are necessary.
Glomerulonephritis with nephrotic Syndrome
An uniform description of NS is not provided, the classification is classified morphologically. Differing etiologies can result in considerable variations in the clinical features, as well as course and prognosis. To establish generally applicable therapeutic concepts and customized treatment for individual patient is the norm, this will be difficult [28]. The different clinical course of this heterogenous disease group render almost impossible to carry out controlled therapy studies. Therapy successes are to be found, as are therapy-produced complications, e.g., infections, sterility, loss of hair, and others. These complications must be weighed against the benefits of immunosuppression. To prevent renal insufficiency and NS must be the aim of the therapy for glomerulonephritis.
Changes in the electrophysiological characteristics of the filtration barriers and of the plasma proteins are the cause of NS. The anionic charge on albumin is retained by the negative charge of the glomerular filter, including the basement membrane and the epithelium, obviously play a decisive role [7]. Hemodynamic changes, such as the increase in venous pressure, can favor the filtration process. Proteinuria can lead to the formation of cellular or fibrous crescent with reciprocal development of RPGN or focal glomerulosclerosis [67]. Proteinuria can also cause overload and dysfunction of tubular epithelial cells, which can result in tubular and interstitial fibrosis. Nephrotic syndrome following COVID-19 vaccination has been reported [68]. The pathogenesis is the activation of angiotensin-converting enzyme-2 receptors, leading to podocyte effacement, and a renal biopsy is necessary to identify this.
Nephrotic syndrome of different GN often reacts to corticosteroids in varying doses, administered over a period of 4-8 weeks. Patients with frequent relapses are also treated with 2-3 mg/kg BW/day cyclophosphamide [27]. Cyclosporine A has also been successfully applied in NS [9]. High doses of immunoglobulin (IgG) are reported for NS. Further therapeutic measures for NS are anticoagulants, thrombocyte inhibitors, ACE inhibitors, immunosuppressive drugs, lipid reducers, biologics and diet [69- 71]. Especially in refractory NS in children and adults rituximab is indicated [72,73]. However, hypogammaglobulinemia is a frequent adverse event after rituximab treatment, also IgG levels slightly increase during B cell depletion. Low serum IgG levels under rituximab treatment in refractory NS are risk factors for the development of hypogammaglobulinemia [74].
Focal sclerosing glomerulonephritis (FSGS) has a less prognosis, and is usually accompanied by NS. Patients with NS have a survival rate of 70% after 6 years, and without NS this rate reaches 85 %. Patients with this form of GN are steroid-sensitive or steroid-non- sensitive. The non-steroid sensitive group of patients could be treated with cyclophosphamide, chlorambucil, cyclosporine A, or other immunosuppressive therapies [28]. Focal sclerosing GN is caused by a variety of factors, however, one type that recurs after transplantation and is associated with circulating factors, could be treated with TPE: After transplantation, as more than 40% of patients with NS have recurrences. In patients with established disease, the glomerular abnormalities include focal and segmental glomerulosclerosis and hyalinosis, although fusion of epithelial- cell foot processes may be the only abnormality early in the course of disease [10].
The AAC of the ASFA has given the FSGN recurrent in kidney transplant the category I with the RG 1B, and the FSGN recurrent in kidney transplantation/steroid resistant in native kidney the category II with the RG 2C, and in steroid resistant in native kidney the category III with RG 2C (Table 1) [10,11]. In native kidneys with FSGN, the treatment is primarily with corticosteroids for at least 6 months prior to trying second-line therapies, such as cyclophosphamide, cases. Patients with recurrent focal glomerulosclerosis have a response to treatment with TPE, lipid apheresis (LA), and IA there may be different circulating factors that alter the glomerular barrier to protein filtration [75].
For FSGN post-transplant, there are no standardized treatment available. The most therapeutic regimens use a combination of immunosuppressive drugs such as cyclophosphamide, biologics, and TPE. Other therapeutic regimens include high- dose cyclosporine, angiotensin converting enzyme inhibitors, indomethacin, and/or tacrolimus. To prevent recurrent FSGN, several sessions of preemptive TPE immediately prior to and following the transplant is recommended [7]. Rituximab and MMF have also been used in conjunction with diagnosed in order to halt the process and maintain renal function [10,11].
With TPE a glycoprotein of molecular weight (MW) of 30-50 kD can be removed that includes profound leakage of albumin when incubated with isolated rat glomeruli. The decrease in serum concentration coincides with improvement in proteinuria. The immediate onset of proteinuria following transplant is mediated by this factor, prophylactic TPE may be instituted in high-risk patients [10,11]. Staphylococcal protein-A columns are recommended in recurrent FSGN in some reports. The procedure is to begin with three daily exchanges followed by at least six more TPE take several weeks to months. Some patients have received longterm monthly exchange as maintenance thersapy [10,11]. Besides TPE and IA, corticosteroids, immunosuppression, calcineurin inhibitors and MMF, or rituximab are recommended [76,77]. Nephrotic syndrome consisting of massive proteinuria, hypoalbuminemia, edema, and hyperlipidemia is a common complication of glomerular disease in children and adults. The annual incidence of NS ranges from 2-7 per 100,000 children, and prevalence from 12-16 per 100,000 [70]. The primary cause of NS is idiopathic. It seems to be a role of the immune system in pediatric minimal change glomerulonephritis (MCGN). Another hypothesis described an association between allergy and MCGN in children. Relapses are triggered commonly by minor infections and occasionally by reaction to be stings or poisoning. Humoral and cellular immunity have been described. The induction of remissions by corticosteroid, alkylating agents, or cyclosporine therapy provides indirect evidence for an immune etiology [10].
Patients with MCGN and massively proteinuria do not have a generalized glomerular leak to macromolecules. The clearance of neutral macromolecules in MCGN is actually less than normal over range of molecular radii. However, the clearance of anionic macromolecules is significantly increased. These suggest that proteinuria results from a loss results from a loss of fixed negative charges of anionic glycosaminoglycan´s in the glomerular capillary wall [11]. The mechanisms through which these charges are lost are not known. Massive albuminuria in NS causes a decrease in intravascular oncotic pressure, which allows extravasation of fluid and hypovolemia, increased aldosterone and antidiuretic hormone secretion, and renal salt and water retention. Minimal change GN can be well treated with customary therapy measures such as prednisolone [78]. Therapy with prednisolone and cyclophosphamide over a period of 8-12 weeks and with LA a significantly rapid faster relief was observed [79,80]. With LA a rapid improvement of hypercholesterolemia in steroid-resistant NS will provide more rapid relief from NS than from steroid therapy alone. Intravenous high dose steroids with alkylating agents, cyclophosphamide, and MMF are recommended in steroid- resistant NS in children, too [81]. Rituximab in the treatment of adult glucocorticoid-dependent/relapsing FSGN can reduce the risk of recurrence and help to decline or discontinue the use of glucocorticoid [82].
Membranoproliferative glomerulonephritis (MPGN) is usually combined with NS and hypertension. The occurrence of NS signifies a poorer prognosis. If corticosteroids or pulse therapy, cyclophosphamide, anticoagulants, and intravenous immunoglobulins are effective is not clarified [28]. Successful seems to be the treatment with protein-A IA in patients with relapsing NS. An indication for TA could be MPGN from cryoglobulinemia.
In peri-membranous glomerulonephritis NS is the main symptom. In acute NS, it is recommended to applicate high dose of prednisolone as a pulse therapy over 3 to 5 days or with 2 mg/kg BW in decreasing dosage for 2 to 3 months. A combination with TPE should be considered especially with the more selective procedures like DFFP, IA, or LA [79,83-85]. In mesangioproliferative glomerulonephritis the symptoms are not usually homogenous. If NS is combined with hypertension the prognosis is poorer. In severe, corticosteroid therapy-resistant cases, a combined therapy with TA and immunosuppression is indicated, regardless of the degree of renal insufficiency [86].
Regular TA treatment seems to be favorably influenced the acute NS. Dysproteinemia and the edema can be improved by larger doses of albumin administration. Therapeutic apheresis is theoretically a way of achieving mainly an improved effect on the basal membrane. The atherogenic risk for patients with NS is reduced by the elimination of cholesterol, LDL, and triglycerides and thus prevent progression. In the management of these diseases, TA should be considered as a useful therapeutic tool [75]. More selective procedures like DFPP, IA, and LA are very useful in the therapy of NS and show a possibility for treating severe cases of NS, if drugs fails. Other renal diseases such as light chain nephropathy, dense deposit diseases and others can be in severe cases and if the conservative therapy has failed treated with TA, too [9].
Hemolytic-uremic syndrome (HUS)
Hemolytic-uremic syndrome is a severe disease that can lead to AKI and often to other serious sequelae, including death. The disease is characterized by microangiopathic hemolytic anemia, thrombocytopenia and AKI. Hemolytic-uremic syndrome is associated with dysregulation of the immune complement system, especially of the alternative pathway [87]. The etiology and pathogenesis of HUS are not completely understood, and the therapy is therefore complicated. The introduction of TPE as a supportive therapy in HUS was very successful in 87% of treated patients. Especially in severe courses, TPE is indicated and is superior to other available therapy [88]. Several studies reported improvements in renal and hematological parameters with the treatment with HMAs, such as eculzumab, or ravulizumab [89,90].
The most patients have infections with enterohemorrhagic E- coli (EHEC). These bacteria can be transmitted through contaminated food, animal and person to person contact. The HUS is causes described as “typical” have to be differentiated since other factors including genetic disorders are of importance. Three different types are subdivided which lead to HUS. Hemolytic uremic syndrome caused by infection, idiopathic HUS (non-Shiga toxin-HUS), and HUS in systemic diseases and after toxin exposure [91].
Spontaneous recovery from HUS has been reported. Various etiological and pathogenetic assumptions have produced different therapy concepts. The total lethality in HUS was first reduced to 20% with the introduction of dialysis [92]. Two-thirds of cases recover without any impairment, if dialysis is administered early enough. In 10 – 20% of cases, however, lasting renal damage occurs. The substitution of plasma or coagulation factors is often necessary due to the severe coagulation problems.
The introducing of TPE, or IA with protein-A in the treatment of HUS was successful [93-96]. A compilation of therapeutic concepts for HUS implemented up to 2009 showed the success of HUS therapy with TPE/HD or IA/HD [88]. Therapeutic apheresis might be more effective than infusions alone, as TA removes potentially toxic substances from blood. In situations that limit the amount of plasma that can be infused, such as renal or heart failure, TPE or TA should be considered first-line therapy. Plasma infusion treatment is contraindicated in S. pneumonia induced non Stx HUS. This treatment may exacerbate the disease because adult plasma contains antibodies against Thomas Friedenreich antigen [97]. In HUS, as shown in various randomized controlled studies, none of the evaluated interventions such as FFP transfusion or dipyridamole, Shiga toxin binding protein and steroids was superior to supportive therapy alone for any outcome [98] . The advantage of TA over other conservative therapeutic procedures is that TA intervenes at an early stage in the pathogenic processes by rapid removing immune complexes and other toxins. Fibrinogen, fibrinogen degradation products, and other high molecular complexes, all can both support and inhibit coagulation, were eliminated by TA. Therapeutic apheresis can be rapidly removed all other toxins produced by bacteria and virus like Shiga-toxin, the pathogenic pathway which follows the activation of the complement system of the factor HF 1with partial HF 1deficiency and all other toxic substances [10]. Therapeutic apheresis seems a reasonable option for the poor prognosis of HUS in adults [88]. The early administration of a monoclonal C5 antibody, eculizumab or ravulizumab, leads in hematologic, kidney, and systemic manifestations in patients to improvements with atypical HUS, even with apparent dialysis dependency [99]. Pre- and posttransplant use of eculizumab is effective in the prevention of atypical HUS recurrence.
The AAC of the ASFA has divided HUS in 3 groups for the treatment with TA. Group 1, diarrhea associated HUS, is a HUS due to complement factor gene mutations has the category II with RG 2C. Group 2 is a HUS due to antibody to factor H, atypical HUS, which has the category I with the RG 2C. Group 3 is the typical HUS < 18 years, MCP mutations, and has the category IV with RG 1C (Table 1) [10,11]. There are no exact guidelines available for the therapy of HUS, due to the various and very different causes, which can lead to HUS.
In HUS, a supportive therapy of TA is indicated, which include control of fluid and electrolyte imbalance, if use of dialysis is required, control of hypertension, blood and plasma transfusion as required. The antibiotic treatment of E.coli O157:H7 colitis may stimulate further verotoxin production and thereby increase the risk of HUS. Hemodialysis or peritoneal dialysis, as required, must be daily. However, untreated HUS in adults and children may progress to end in organ damage [88]. Platelet transfusion may actually worsen outcome. Therapeutic plasma exchange or IA is performed daily until the platelet count is in a normal range. The replacement fluid in TPE consists of a 5% human albumin electrolyte solution in 30-70% and FFP in remainder. A 1-1.5 TPV should be the exchange volume per treatment depending on the severity of the disease. Therapeutic plasma exchange reverse the ongoing platelet consumption. In IA no replacement fluid is necessary, only between the treatments FFP or coagulation factors may be transfused if required. Hemodialysis treatment can be combined with TA.
Escherichia coli (O104:H4) was in Germany in May to July 2011 with 3,167 without HUS and 16 deaths in patients, and 908 with HUS and 34 deaths [100]. With HUS, 241 were treated with TPE and 193 patients with a combination of TPE and eculizumab. The treatment strategy depended on the severity of the diseases [101]. The combination of TPE and eculizumab seems to be prudent and necessary prior to establishing new treatment guidelines. Eculizumab or ravulizumab, two HMAs, were introduced successfully in the treatment of HUS [99,102,103]. Especially in renal graft survival in atypical HUS, prophylactic application of eculizumab was very successful [104,105]. The conversion from TA to eculizumab therapy should be considered in patients with HUS who show an incomplete response to TA. Careful attention should be paid to meningococcal infection, eculizumab-related infusions reactions and allergic with administration of eculizumab [106]. The combination of TA, HMAs, and immunosuppression treatments provides benefits in HUS. However, further multicenter, randomized trials are necessary to find the best treatment in HUS.
Kidney Transplant Rejection
In end-stage renal disease, a kidney transplantation is the decisive alternative to permanent dialysis. Rejection of the transplanted kidney is a severe problem. In the last decades, different therapeutic interventions to delay or prevent rejection exist, including steroids, IVIG, immunosuppressives, cyclosporine A, triple drug, OKT3, other new developed immunosuppressive therapies, and HMAs. Infections and rejection reactions are the most frequent complications of transplantation [107,108]. Acute kidney transplant rejection is an indication for TA [107,109].
Immunological problems like performed donor-specific abs or a high degree of immunization complicate the outcome of donor transplantation. The therapeutic success of transplantation the medication. In the management of rejection crisis due to performed specific abs or high degree of immunization, TA is indicated [108]. The outcome of donor transplantation is complicated by immunological problems like high postoperatively antibody- mediated rejection or drug-related side effects of the medication. Acute allograft rejection is one of the important complications after renal transplantation, and it is a deleterious factor for long-term graft survival [28]. Rejection is a complex pathophysiologic process, which has been explained by transcriptome and proteome in RNA transcripts and proteins level respectively [110,111]. Therapeutic strategies include a primary avoidance of immunization, carful patient selection, a meticulous immunological workup and a proper follow up and TA as improved therapy [112,113].
Different therapy protocols were developed for ABO-incompatible kidney transplantation and the procedure has gained widespread acceptance and has implemented in most transplant centers [110,111]. These protocols include immunosuppression of tacrolimus, mycophenolate and steroids together with induction therapy with an IL-2-receptor blocking agent. The CD 19/20-positve pre-B cells with a single infusion of rituximab four weeks prior to transplantation and in a second step, the already existing abs are depleted by using TPE or IA. The isoagglutinine antibodies against the donor can be eliminated. Novel sensitization and production of abs is thereby efficiently prevented [113,114]. The disadvantage of TPE is the elimination of physiological protein, the limitation to 1-1.5 TPV as treating dose and the potential for infectious complications such as HIV or hepatitis B or C by using plasma as substitution. Immunoadsorption with unselective IgG columns is therefore used from different groups. Patients with performed HLA abs, i.e., a high percentage of panel reactive abs, accumulate on the waiting list for kidney transplantation and can experience a substantially longer waiting time [108,113]. Center specific desensitization protocols were developed in order to transplant these highly immunized patients within a reasonable period.
Problematic is the transplantation procedure with deceased donor organs as the time for pre-conditioning of the recipient is extremely limited and the accompanying procedures are difficult to perform in time. Different protocols were established to desensitize the recipient, if transplantation from a living donor is planned. These strategies require an intensive procedure, consisting of the administration of IVIG, intensified immunosuppression, HMAs, pre- and postoperative TA and carry a higher risk for ab-mediated rejection [108,115-117]. Therapeutic IA columns with a selectivity for immunoglobulins would be considered the best option. To deplete to recipient of the donor-specific antibody (DSA)- and/or anti-HLA titer, some treatments are usually needed.
Severe dysfunction with a high risk of allografts loss follows acute ab rejection of organ allografts. In antibody-mediated rejection (AMR), HLA abs are involved [118]. Therapeutic apheresis accompanied by T cell depletion (ATG, ALG, or OKT3) conversion to a tacrolimus-based immunosuppression and pulsed steroids, are used to limit the interstitial and vascular damage [116]. The use of IA targeted against IgG has been successfully. In a five year survival trial, IA was superior to TPE and DFPP [119]. However, to give general recommendation about the number of TA sessions and the best immunosuppressive therapy is not possible. To monitor the abs titer during treatment, screening for donor-specific abs should be performed. About 10 TA sessions with daily treatments initially followed by TA every other day can be necessary in patients with vascular rejection (Banff IIb-III AMR [108,115].
With the use of calcineurin in the inhibitor or mechanistic target of Rapamycin (mTOR) inhibitors or acute vascular rejection, recurrence or de novo thrombotic microangiopathic (TMA) in the transient patient is observed. Infections such as HIV, CMV, Para virus B 19, an inhibited or decreased activity of the von Willebrand factor cleaving metalloprotease ADAMTS13 or mutations in complement receptors may also trigger microangiopathy with either limited or systemic manifestation [108].
If switching to a different immunosuppressive regimen or treatment of an underling infection does not lead to an improvement of the TMA, TA can be attempted to ameliorate the course of the disease and subsequent graft damage [120]. In TPE the treated volume is a 1-1.5 TPV with an electrolyte human albumin solution (5%) and/ or FFP as substitution solution and anticoagulation with heparin on a daily basis until platelet count and lactate dehydrogenase have normalized. Up to 50% of patients demonstrate a prompt exacerbation if daily TA is stopped. The recurrence rate can be reduced by continuation of TA on an alternative day strategy for at least two additional treatments. However, TMA reduces graft survival both in recurring or de novo TMA and treatment might not alter the progression of the disease [108].
Goodpasture syndrome and/or anti-GBM disease can occur de novo in patients following transplantation or as a manifestation of underlying the rare Alport syndrome [121,122]. The recipient´s immune system, which is exposed to a collagen component carried by the transplanted organ, is lacking in Alport patients, and therefore, the patient might develop antibodies against this antigen in the glomerular basement membrane, and these antibodies may then induce post transplantation anti-GBM disease.
The strategy of the treatment of this disease and of de novo disease is identical to non-transplanted. Therapeutic apheresis has been shown to deplete the patient effectively of antibodies and halt disease progression [123,124]. With TA, a rapid removal of the antibodies with daily sessions is possible. To antibody titer measurements, the treatment frequency should be tapered later. However, TA treatment is accompanied by an intensified immunosuppressive regimen to suppress further antibody formation [108]. New complement blockers raises hope to significantly reduce the abmediated rejection, and nephropathy recurrence on outcomes after kidney transplantation [125]. From kidney transplantation in adults with focal sclerosing glormerulosclerosis only some information are available about long-term results. Primary FSGN recurs with uncertain incidence after kidney transplantation, presumably 20 %. Therapeutic apheresis has been successfully applied in patients with recurrent FSGN [126]. A dramatic but usually transient reduction in proteinuria has been observed, if patients treated with TA. This effect was larger with the use of IA and in combination of cyclophosphamide [108].
Therapeutic apheresis is an important part of different transplantation therapy strategies for several conditions such a as AMR or ABOi transplantation is accepted. Therapeutic apheresis to develop strategies to provide the best organ replacement to patients with high degree of immunization or performed FDSA thereby expanding the use of living donation. Therapeutic plasma exchange is more and more replaced by the more selective methods of immunoadsorption. The AAC of the ASFA describe the ab-mediated rejection and HLA desensitization as follows and give the AMR renal transplant recipients and desensitization living donor due to donor specific HLA ab the category I with RG 1B. The desensitization high panel-reactive antibody (PRA) deceased donor has the category III with the RG 2C (Table 1) [10,11]. Antibody-mediated rejection affects less than 10% of the renal allografts. Recipients at increased risk include those with previous transplant and high panel reactive abs [10].
To prevent and treat acute allograft rejection, new immunosuppressive drugs are continually being developed. All transplant recipients are placed on immunosuppressive therapy, however, individuals with a high likelihood of acute rejection, including those with HLA abs and recipients of cadaveric organs receive regimens that are more intensive. An optimal regimen has yet not to be defined, however, include besides cyclosporine, tacrolimus, mycophenolate mofetil, azathioprine, and antilymphocyte globulin [127-129]. Other report show that belatacept may be an alternative to calcineurin inhibitors and may contribute to improve long-term metabolic and allograft outcomes in kidney transplant recipients [130]. More and more HMAs are successfully introduced, such as rituximab, eculizumab, etc. [10,11]. Clazakizumab, alemtuzumab, or nivolumab are newer HMAs and are introduce successfully in the treatment of AMR in kidney transplantation [127-129].
The rationale for TA is that AMR and DSA, which generated after transplantation, can be removed with TPE, DFPP, lymphoplasmapheresis, and IA [10]. To lower ab titer below a critical threshold, TPE is used. In preparatory regimen for ABOi renal transplantation in addition to other immunosuppressive/ immunomodulatory drugs therapies, TPE is included. To improve anti-rejections, improved detection of DSA, and improved definition of AMR using the Banff criteria, these therapy regimens are used. Previously there was a high graft loss rate with acute vascular rejection, current regimen, which include TPE, have a graft survival rate of 70-80% [7]. To remove HLA abs, TA can also be used prior to transplantation. Therapeutic apheresis is always used in combination with immunosuppressive drugs pretransplant until cross-match is negative, and is continued usually postoperatively and reinitiated in cases where AMR occurs. To obtain a negative cross-match depends on the DSA titer. Approximately five TA sessions preoperatively will allow the titer of ≤ 32 to become negative. The risk of AMR is approximately 30% with a small number of graft losses. Only in highly selected patients, the desensitization protocols should be used [29].
The therapy of the patients should be started with immunosuppressive drugs prior to initiate TA to limit antibody re- synthesis. There must be a correlation between the number of TPE needed pre-operative to obtain a negative crossmatch and the ab titer in the desensitization protocols [11]. The exchange volume is 1-1.5TPV and the replacement solution can be a 5% human- albumin- electrolyte solution and/or FFP. For a minimum of three procedure, TPE is also performed postoperatively [11,29].
More investigations and controlled trials will be necessary and will show the importance of TA in the therapy strategies, but the financial aspects of TA are matter of regional negotiation and preference. The transplant centers should define their needs for a standard reimbursement and to try to limit price variation of this very expensive therapy to simplify reimbursement [108]. Besides TA, immunosuppressive drugs, new HMAs, and other inhibitors should be individualized introduced in patients with acute or chronic kidney rejection, and this must be clarified in further studies [131,132].
Closing Remarks
Since more than 45 years hollow fiber modules were introduced in the treatment of various diseases. More and more, the pathogenetic relevance of autoantibodies could be defined in different immunologic diseases, and disease-specific adsorbers and new hollow fiber modules as second membranes have been developed, more immunologic and non-immunologic diseases can be treated with TA. Vascular access plays an important role besides the physical problems and technical ones such as the apparatus required [133]. Important is if TA is indicated to start it early as possible in the treatment with an adequate exchange volume of 40- 50 ml/kg BW and a lowest possible extracorporeal volume [134]. An adequate substitution solution is only necessary in TPE. During and between the TA sessions, the patient must be monitored. Special attention is important to circulation, consciousness, coagulation status, and blood count. Sterile procedure must be adhered, to prevent catheter infection or sepsis by using a large-bore catheter placed in a central vein [135]. During the acute phase of the disease, TA appears to be benefit [136]. Therapeutic apheresis is used in renal diseases such as the AKI, the rare NSF, various forms of glomerulonephritis, HUS, and renal transplant rejections. Only a few prospective controlled trials are available to allow definitive conclusions. In RPGN (ANCA associated) with dialysis dependency (Cr > 6 mg/dL) and in RPGN with diffuse alveolar hemorrhage (anti-glomerular basement membrane disease) TA is indicated with immunosuppression therapy. In RPGN with dialysis independence, TA is only indicated in severe cases if the immunosuppressive therapy has failed. The efficacy of TA as an adjunct to immunosuppressive therapy was shown in some studies in approximately 60% of patients with RPGN present with crescentic GN (pauci-immune RPGN) with few or absent deposits. One type of FSGN that recurs after transplantation has circulating factors and can be treated with TA. A further indication for TA could be the MPGN from cryoglobulinemia. The rationale for TA in HUS is discussed controversially, and the treatment strategy is dependent of the disease severity. Therapeutic apheresis in combinations with HMAs such ecumlizumab seems to be prudent. In renal transplantation, TA is indicated in ABO compatible ab-mediated rejection, desensitization, living, and positive crossmatch due to donor specific HLA ab. In ABO incompatible renal transplantation, TA is indicated for desensitization live donors and in humoral rejection. Besides corticosteroids, IVIG, and immunosuppressive drugs has been established as first-line therapy in different renal diseases. Never therapy modalities with HMAs such as rituximab, eculizumab, belimumab, clazakizumab and others showed clinically improvement in severe and refractory renal disorders. In kidney transplant rejection treated with nonbelatacept (MMF, tacrolimus and sirolimus) and belatacept- based (MMF, tacrolimus and belatcept), the patients has benefit of this therapy [132].
All mentioned TA methods are still technically complicated and very expensive. A reduction in costs is a valid demand in view of the scarce resources available in the healthcare systems. Physicians are committed to helping al patients entrusted to them to the best of their knowledge, and this means that medical treatment, and particularly the apheresis process, must become affordable. To physicians, health organization, and above all to the manufactures, this demand represents a great challenge. For all mentioned diseases the quotient relevant for cost-effectiveness assessment (cost of treatment – cost saved): (improvement in life quality) must discussed and calculated exactly by all involved persons. Every effort should be made to delay the progression of acute and chronic diseases. Therapeutic apheresis is clearly an important tool in treatment of many complex conditions now and in the future [137]. However, further studies are necessary to see which is the best therapy option.
Conclusion
Therapeutic apheresis in the treatment of renal diseases expands the clinical practice of nephrologists. More than 80% of the treated patients could be healed with TA, IVIG, immunosuppression drugs and/or HMAs. The most of renal diseases such as AKI, NSF, various RPGN diseases, HUS and renal transplantation rejections has been improved under the treatment with TA and immunosuppression drugs. Especially in severe renal failure, TA with immunosuppression, steroids, cytotoxic agents and/ or HMAs is indicated. However, all mentioned TA methods are still technically complicated and very expensive. A reduction in costs is a valid demand in view of the scarce resources available in the healthcare system. More prospective controlled studies are necessary.
References
- Bambauer R, Schiel R, Lehmann B, et Therapeutic Apheresis Overview. ARPN J Sci Technol. 2012; 2: 399-422.
- Malchesky PS, Bambauer R, Horiuchi T, et al. Apheresis Technologies An International Perspective. Artif Org. 1995; 19: 315-323.
- Hafer C, Golla P, Gericke M, et Membrane versus centrifuge based therapeutic plasma exchange a randomized prospective crossover study. Int Urol Nephrol. 2016; 48: 133-138.
- Kaplan AA. Why nephrologists should perform therapeutic plasma Dial Transplant. 2009; 38: 65-70.
- Madoer F, Larzarus JM, Brady HR, et el. Therapeutic plasma exchange in renal J Am Soc Nephrolog. 1996; 7: 367- 386.
- Bambauer New Aproaches in the treatment of Acute Kidney Injury. Ther Apher Dial. 2009; 10: 248-253.
- Bambauer R, Bambauer C, Latza R, et Therapeutic apheresis in nephrology. Clin Nephrol Urol Sci. 2014.
- Bambauer R, Latza R, Bambauer C, et Therapeutic apheresis in autoimmune diseases. Rhematol Res Rev. 2013; 5: 93-103.
- Bambauer R, Latza R, Burgard D, et Therapeutic Apheresis in Hematologic Autoimmune and Dermatologic Diseases with Immunologic Origin. Ther Apher Dial. 2016; 20: 433-452.
- Schwarz A, Padmanabhan A, Aqui N, et al. Guidelines on the use of Therapeutic Apheresis in Clinical Practice Evidence based Approach from the writing Committee of the American Society for Apheresis the Seventh Special J Clin Apher. 2016; 31: 149-162.
- Padmanabhan A, Connelly Smith L, Aqui N, et al. Guidelines on the use of Therapeutic Apheresis in Clinical Practice Evidence based Approach from the writing Committee of the American Society for Apheresis Eighth Special Issue. J Clin 2019; 34: 171-354.
- Bambauer R, Latza R, Burgard D, et Autoimmune Disorders treated with Therapeutic Apheresis. Immunol Res Ther J. 2017; 1: 111-131.
- Web S, Dobb G. ARF or AKI Its now acute kidney injury. Anaest Intensive Care. 2007; 35: 843-844.
- Jörnes A, Frei Akutes Nierenversagen. Internist. 2001; 42:379-403.
- Kribben A. Pathogenese des akuten Nierenversagens. Mitt Klin 2004; 23: 33-34.
- Albuerque P, Meneses G, Silva Junioe GB, et Toxin related Acute Kidney Injury In nephrology and Public Health Worldwide. Contrib Nephrol. 2021: 199: 131-142.
- Naleesso F, Stefanelli FC, Di Vico V, et al. The Supporting Role of Combined and Sequential Extracorporeal Blood Purification Therapies in COVID 19 patients in Intensive Care Biomedicines. 2022; 10.
- Rosner MH, Perazella Acute kidney injury in the patient with cancer. Kid Res Clin Pract. 2019; 38: 295-308.
- Bambauer R, Schiel Therapeutic Apheresis in Acute Pancreatitis due to Hypertriglyceridemia. Ann Cardiol Cardiovascul Dis. 2017; 2: 1014-1022.
- Bardin T, Richette Nephrogenic systemic fibrosis. Curr Opio Rheumatol. 2010; 22: 54-58.
- Baron PW, Cantos K, Hillebrand DI, et Nephrogenic fibrosing dermopathy after liver transplantation successfully treated with plasmapheresis. Am J Dermatopathol. 2003; 25: 204-209.
- Hofmann JC, Reynolds SL, Kiprov Nephrogenic fibrosing dermopathy response to plasma exchange. J Clin Apher. 2005; 20: 12-13.
- Gillier M, Cozzio A, Burg G, et Successful treatment of these cases of nephrogenic fibrosing dermopathy with extracorporeal photopheresis. Br J Dermatol. 2005; 152: 531- 536.
- Mathus K, Morris S, Deighan C, et Extracorporeal photopheresis improves nephrogenic fibrosing dermopathy nephrogenic systemic fibrosis three cases reports and review of literature. J Clin Apher. 2008; 23: 144-150.
- Poisson JL, Low A, Park YA, et The treatment of nephrogenic systemic fibrosis with therapeutic plasma exchange. J Clin Apher. 2013; 28: 317-320.
- Farooqi S, Mumtaz A, Arif A, et The Clinical Manifestation and Efficacy of Different treatments Used for nephrogenic Systemic Fibrosis A Systematic Review. Inter J Nephrol Renovascul Dis. 2023; 16: 17-30.
- Bambauer R, Latza R, Schiel R, et al. Therapeutic Apheresis in Immunologic Renal and neurologic Diseases. Ther Aper 2017; 21: 6-21.
- Bambauer R, Schiel Therapeutic Apheresis and Immunosuppression in Immunologic Diseases A Review and Own Observations. Clin Immunol Res. 2021; 5: 1-36.
- Bambauer R. Therapeutic Apheresis and Immunosuppression in Renal Diseases. J Clin Res Reports. 2021; 7: 1-15.
- Morris A, Geetha Advances in remission induction therapy for Anca assosciated vasculitis. Best Prat Res Clin Rheumatol. 2023; 101828.
- Cortzar FB, Cerda J, Dhanai R, et Avacopan in patients with rapidly progressive glomerulonephritis requiring dialysis. Kid Intern Rep. 2023; 8: 1687-1691.
- Menon S, Bagga A. Rapidly Progressive Glomerulonephritis. Ped Kid 2023: 575-590.
- Stummvoll G, Aringer M, Handisurya A, et Immunoadsorption in Autoimmune Diseases Affecting the Kidney. Kid Sem Nephrol. 2017; 37: 478-487.
- Walsh M, Merkel PA, Peh CA, et al. Plasma exchange and glucocorticoid dosing in the treatment of antineutrophil cytoplasma antibody associated vasculitis Trials. 2013; 14: 73.
- Balogun RA, Sanchez AP, Klingel R, et al. Update to the ASFA guidelines on the use of therapeutic apheresis in ANCA associated J Clin Apher. 2020; 35: 493-499.
- Walsh M, Merkel PA, Peh CA, et al. Plasma Exchange and Glucocorticoids in severe ANCA Associated Vasculitis. N Engl J 2020; 382: 622-631.
- Kaplan AA, Szpirt Should we still use therapeutic plasma exchange for rapidly progressive glomerulonephritis in ANCA associated vasculitis. Kid Dial. 2022; 2: 399-406.
- Guo S, Mühlfeld AS, Wietecha TA, et Deletion of acting Fcγ receptors does not confer protection in murine cryoglobulinemia associated membranoproliferative glomerulonephritis. Am J Pathol. 2009; 175: 107-116.
- Clynes R, Dumitru C, Ravetch JV, et Uncouplin of immune complex formation and kidney damage in autoimmune glomerulonephritis. Science. 1983; 279: 1052-1054.
- Bambauer R, Bambauer C, Latza R, et Therapeutic apheresis in nephrology. Clin Nephrol Urol Sci. 2014.
- Tensaki M, Takashi H, Sato R, et al. Successful Treatment with Multitarget Therapy of myco phenmolate Mofetil and Tacrolimus for Cyclophosphamide resistant Antineutrophil Cytoplasmic Developed Independently of Systemic Lupus Erythematosus. Clin Rheumatol. 2021; 27: 79-80.
- Norris M, Remuzzi C3G and Ig MPGN treatment standard. Nephrol Dial Transplant. 2023.
- Reggiani F, LÍmperio V, Calatroni M, et al. Goodpasture syndrome and anti glomerular basement membrane disease. Clin Exp Rheumatol. 2023; 41: 964-974.
- Nasim W, Naidas Clinicopathologic Predictors of Prognosis in Anti Glomerular basement Membrane Disease Can We Do Better. ASN Kidney News. 2023.
- Lin J, Markowitz GS, Valeri AM, et al. Renal monoclonal immunoglobulin deposition disease. The disease spectrum. Am Soc 2001; 12: 1482-1492.
- De Lind van Wijngaarden RA, Hauser HA, Wolterbeck R, et Clinical and histologic determinants of renal outcome in ANCA associated vasculitis A prospective analysis of 10 patients with severe renal involvement. J Am Nephrol. 2006; 17: 2264-2272.
- De Lind van Wijngaarden RA, Hauser HA, Wolterbeck R, et for the European Vasculitis Group. Changes of renal recovery for dialysis dependent ANCA associated glomerulonephritis. J Am Soc Nephrol. 2007; 18: 2189-2199.
- Uhlin F, Szpirt W, Kronbichler A, et Endopeptidase Cleavage of Anti Glomerular Basement Membrane Antibodies in vivo in Severe Kidney Disease An Open Label Phase 2a Study. JASN. 2022; 33: 829-838.
- Prrendecki M, Pusey C. Plasma exchange in antiglomerular basement membrane Presse Medicale. 2019; 48: 328- 337.
- Ting JA, Barbir ED, Levin A, et Double positive anti glomerular basement membrane antibody and myeloperoxidase antineutrophil cytoplasmatic autoantibody associated glomerulonephritis post COVID 19 mRNA A case series of 4 patients. Can J Kid Health Dis. 2023; 10.
- Guo S, Mühlfeld AS, Wietecha TA, et Deletion of acting Fcγ receptors does not confer protection in murine cryoglobulinemia associated membranoproliferative glomerulonephritis. Am J Pathol. 2009; 175: 107-116.
- Clynes R, Dumitru C, Ravetch JV, et Uncoupling of immune complex formation and kidney damage in autoimmune glomerulonephritis. Science. 1983; 279: 1052-1054.
- Bambauer R, Schiel Therapeutic Apheresis in renal diseases with immunologic origin. Ann Immunol Immunother. 2020; 2: 000114.
- Tensaki M, Takashi H, Sato R, et al. Successful Treatment with Multitarget Therapy of myco phenmolate Mofetil and Tacrolimus for Cyclophosphamide resistant Antineutrophil Cytoplasmic Developed Independently of Systemic Lupus Clin Rheumatol. 2021; 27: 79-80.
- Norris M, Remuzzi C3G and Ig MPGN treatment standard. Nephrol Dial Transplant. 2023.
- Jayne DRW, Gaskin G, Rasmussen N, et European Vasculitis Study Group. Randomized trial of plasma exchange or high dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J Am Soc Nephrol. 2007; 18: 2180- 2188.
- Hasegawa M, Kawmura N, Kasugai M, et Cytapheresis for the treatment of myeloperoxidase antineutrophil cytoplasmatic anti associated vasculitis. Reports of 5 cases. Ther Apher. 2007; 20: 261-270.
- Liu X, Xia M, Liu D, et Efficacy of protein A immunoadsorption and therapeutic plasma exchange in ANCA associated vasculitis with severe renal involvement a retrospective study. Ann Med. 2023; 55.
- Ewert BH, Jeannette JC, Falk RJ, et al. Anti myeloperoxidase antibodies stimulate neutrophils to damage human endothelial Kid Int. 1992; 41: 375-383.
- Cole E, Caffran D, Magli A, et al. A prospective randomized trial of plasma exchange as additive therapy in idiopathic crescentic glomerulonephritis. The Canadian Apheresis Study Am J Kid Dis. 1992; 20: 261-270.
- Cascian AL, Jayne DRW. Role of plasma exchange in the treatment of primary vasculitis. Int. J Clin Rheumatol. 2010; 5: 339-344.
- Hayes JS, Chang J, Abdel Rahmann EM, et al. Therapeutic plasma exchange for related conditions in the elderly Ten year experience in one Sem Dial. 2012; 25: 159-164.
- Hayes JS, Chang J, Abdel Rahmann EM, et al. Therapeutic plasma exchange for related conditions in the elderly Ten years experience in one Sem Dial. 2012; 25: 159-164.
- Walsh M, Catapano F, Szpirt W, et al. Plasma exchange for renal vasculitis and idiopathic rapidly progressive glomerulo- A meta analysis. Am J Kid Dis. 2011; 57: 566-574.
- Magri SJ, Ugarte Gil MF, Brance ML, et al. Pan American League of Associations for Rheumatology Guidelines for the treatment of ANCA associate vasculitis. The Lancet. 2023; 5: E483-E494.
- Watanabe H, Yamana H, Okada A, et al. Therapeutic plasma exchange for anti glomerular basement membrane disease with dialysis dependent kidney failure without diffuse alveolar hemorrhage. J Nephrol. 2023; 36: 2317-2325.
- Ponticelli C, Moroni Nephrotic syndrome pathophysiology and consequences. J Nephrol. 2023.
- Parikh C, Upadhay, Patel S, et Nephrotic syndrome following COVID19 vaccination a systematic review. J Nephrol. 2023.
- Habashy D, Hodson EM, Craig JC, et al. Interventions for steroid resistant nephritic A systemic review. Pediatr Nephrol. 2004; 18: 906-912.
- Bagga A, Mantan Nephrotic syndrome in children. Ind J Med Red. 2005; 120: 13-19.
- Zheyi D, Jianhui Z, Zhonggao X, et Efficacy and Safety of Mizoribine for the Treatment of refractory Nephrotic Syndrome protocol for a Multicenter Controlled Open label Randomized Controlled Trial. JMIR Res Prot. 2023; 12.
- Chanm EY, Yap DY, Collucci M, et al. Use of Rituximab in Childhood Idiopathic nephrotic Syndrome. Clin J Am Soc 2023; 18: 533-548.
- Ma X, Fang L, Shend L, et Rituximab treatment fort refractory nephrotic syndrome in adults a multicenter retrospective study. Ren Fail. 2023; 45.
- Inoki Y, Kamei K, Nishi K, et al. Incidence and risk factors of rituximab associated hypogammaglobulinemia in patients with complicated nephrotic syndrome. Pediat Nephrol. 2022; 37: 1057-1066.
- Ronco PM, Alyanakian MA, Mougenot B, et al. Legit chain deposition disease A model of glomerulosclerosis defined at the molecular Am Soc Nephrol. 2001; 12: 1558-1565.
- AL Shami HR, Shaheen I, Aziz D, et al. Management of recurrent focsal segmental glomerulosclerosis post renal Transplant Rev. 2022; 26: 100675.
- De cos M, Meliambro K, Campbeli KN, et Novel Treatment paradigms Focal Segmental Glomerulosclerosis. Kid Inter Rep. 2023; 8: 30-35.
- Matto TK, Mahmoud MA. Increased maintenance cortico- steroids during upper respiratory infection decrease the risk of relapse in nephritic Nephrol. 2000; 85: 342-348.
- Musco E, Mune M, Fujii Y, et The Kansai FGS LDL apheresis FLAT Study Group. Nephron. 2001; 89: 408-414.
- Nakayama M, Katafuchi R, Yanase T, et Steroid responsiveness and frequency of relapse in adult onset minimal change nephrotic syndrome. Am J Kid Dis. 2002; 39: 503-512.
- Alshaya HO, AL Maghrabi JA, Kari JA, et al. Intravenous pulse cyclophosphamide is it effective in children with steroid resistant nephrotic Pediatr Nephrol. 2006; 18: 1143-1146.
- Liuwei W, Lu Y, Yulin W, et al. Rituximab treatment of adults with primary focal segmental glomerulosclerosis. Sci Rep. 2023; 13: 6740.
- Esnault VL, Besnier D, Testa A, et Effect of protein A immunoadsorption in nephrotic syndrome of various etiologies. J Am Soc Nephrol. 1999; 20: 2014-2017.
- Russo GE, Nonello M, Bunco B, et al. Nephrotic syndrome and plasmapheresis. Int J Artif 1999; 23: 111-117.
- Straube R, Gäxkler D, Thiele A, et al. Membrane differential filtration is safe and effective for the long term treatment of Refsum syndrome an update of treatment modalities and pathophysiological Transf Apher Sci. 2003; 29: 85-91.
- Haas M, Godfin Y, Oberbauer R, et Plasma immunoadsorption treatment in patients with primary focal and segmental glomerulonephritis. Nephrol Dial Transplant. 1998; 13: 2013-2017.
- Yokoo T, Tanabe A, Yoshida Y, et al. Antibody recognition of complement factor H reveals a flexible loop involved in atypical hemolytic uremic syndrome pathogenesis. Biol Chem. 2022; 298: 101962.
- Bambauer R, Latza R, Schiel R, et al. Therapeutic apheresis in the treatment of hemolytic uremic syndrome due to pathophysiologic Ther Apher Dial. 2011; 15: 10-19.
- Medeiros de Souza R, Mendes Correa BH, Moreiro Melo PH, et al. The treatment of atypical hemolytic uremic syndrome with eculizumab in pediatric patients a systematic review. Pediat 2023; 38: 61-75.
- Lo SH, Aggio D, Gatta F, et al. P 599 Real world clinical practices in treatment discontinuation with complement C inhibitors for atypical hemolytic uremic syndrome A global survey of healthcare Hemasphere. 2023; 7: e9823510.
- Zimmerhackl LB, Verweyen H, Gerber A, et Das Hämolytisch urämische Syndrom. Dtsch Ärztbl. 2002; 99: A197-A203.
- Bläkert F, Altrooge H, Hellwege HH, et Dialysebehandlung des schweren hämolytisch urämischen Syndrom. Dtsch Ärztebl. 1978; 103: 1229-1233.
- Spencer CD, Crane FM, Kumar JR, et Treatment of postpartum hemolytic uremic syndrome with plasma exchange. JAMA. 1982; 247: 2808-2809.
- Gillor A, Roth B, Bulla M, et Plasmapherese bei der Behandlung von Kindern mit hämolytisch urämischen Syndrom. Nieren Hochdruckkrankh. 1986; 15: 118-123.
- Bambauer R, Jutzler GA, Jesberger HJ, et Therapeutischer Plasmaaustausch beim hämolytisch urämischen Syndrom. Dtsch Med Wschr. 1988; 113: 1245-1249.
- Dittrich E, Schmaldienst S, Derfler K, et Plasmaaustausch und Immunadsorption. Wien Klin Wschr. 2007; 2: 39-54.
- Kaplan AA. Therapeutic apheresis for cancer related uremic Ther Apher. 2001; 4: 201-206.
- Michael M, Elliot EJ, Ridley GF, et al. Interventions for hemolytic uremia and thrombotic thrombocytopenic purpura. Crochane database Sys 2009; 53: 259-272.
- Gurevich E, Landau Pharmacological Management of Atypical Hemolytic Uremic Patients Current and Future. Pediatric Drugs. 2023; 25: 193-202.
- Rasco DA, Webster DR, Sahl JW, et al. Origins of the E coli strain causing outbreak of hemolytic uremic syndrome in N Engl J Med. 2011; 365: 709-717.
- Kielstein JT, Beutel G, Feig S, et al. Best supportive care and therapeutic plasma exchange with or without eculizumab in Shiga toxin producing E coli O104 H4 induced hemolytic uremic syndrome an analysis of the German STEC HUS Nephrol Dial Transplant. 2012; 22: 266-272.
- Tomazos I, Halswell AJ, Galaland S, et Comparative efficacy of ravulizumab and eculizumab in the treatment of atypical hemolytic uremic syndrome An indirect comparison using clinical trial data. Clin Nephrol. 2022; 97: 261-272.
- Thomas K, Ananthula A, Lopez Flores R, et The use of eculizumab for the treatment of Atypical hemolytic uremic syndrome in an Academic Hematology Center. Permanente J. 2023; 27.
- Glover EK, Smith Jackson K, Brocklebank V, et al. Assessing the impact of prophylactic eculizumab on renal graft survival in atypical hemolytic uremic Transplantation. 2023; 107: 994-1003.
- Balwani MR, Posari A, Gurjjor P, et al. Kidney transplant outcomes in patients with atypical hemolytic uremic Transplant Proc. 2023; 55: 1312-1315.
- Ashida A, Matsumura H, Shimono A, et al. Comparison of outcomes after plasma therapy or eculizumab in pediatric patients with atypical hemolytic uremic Clin Exper Nephrol. 2023; 27: 161-170.
- Mao Y, Bai J, Chen J, et A pilot study of GC MSbased serum metallic profiling of acute rejection in renal transplantation. Transplant Immunol. 2008; 19: 74-79.
- Teschner S, Kurschat C, Burst V, et al. Therapeutic apheresis in transplantation overview and critical evaluation of available modalities in respect to indications evidence and Transplantationsmedizin. 2010; 22: 266-272.
- Gloor JM, Winters JL, Cornell LD, et al. Baseline donor specific antibody levels and outcomes in positive crossmatch kidney Am J Transplant. 2010; 10: 582-588.
- Ichimaru N, Takahara Japan s experience with living donor kidney transplantation across ABO barriers. Nat Clin Pract Nephrol. 2008; 4: 682-692.
- Sawada T, Fuchinoue S, Teraoke S, et Successful AI to O ABO incompatibility kidney transplantation after a preconditioning regimen consisting of anti CD20 monoclonal antibody infusions splenectomy and double filtration. Transplantation. 2009; 74: 1207-1214.
- Donauer J, Wilpert J, Geyer M, et ABO incompatible kidney transplantation using antigen specific immunoadsorption and rituximab a single center experience. Xenotransplantation. 2006; 13: 118-114.
- Scharma A, Bummerts J, Gomez Navarrro D, et Clearance for monoclonal antibody CP 675 206 by therapeutic plasma exchange or plasmapheresis. J Clin Oncol. 2007; 25: 1515-1519.
- Franz HJC, Rahmel A, Doxiades II, et al. Enhanced kidney allocation to highly sensitized patients by an acceptable mismatch Transplantation. 2009; 88: 447-452.
- Jordan SC, Tyan D, Stablein D, et Evaluation of intravenous immunoglobulin as an agent to lower allosensitization and improve transplantation in highly sensitized adult patients with end stage renal disease report of the NIH IG02 trial. J Am Soc Nephrol. 2004; 15: 3256-3262.
- Vo A, Lukovsky M, Toyoda M, et Rutiximab and intravenous immunoglobulin for desensitization during renal transplantation. N Engl J Med. 2008; 359: 242-248.
- Hullegie Peelen DM, van der Zwan M, Clahsen van Groningen MC, et al. Clinical and Molecular Profiling to Develop a Potential Prediction Model for the Response to Alemtuzumab Therapy for Acute Kidney Transplant Rejection. Clin Pharmacol Therap. 2022;111: 1155-1164.
- Venetz JP, Pascual M. New treatments for acute humoral rejection of kidney allografts. Inform Healthcare. 2007; 16: 625-631.
- Caliskan Y, Mirioglu S, Dirim AD, et al. A comparison of methods of plasmapheresis for the treatment of late antibody mediated rejection in kidney transplant Ther Apher Dial. 2022; 27; 428-434.
- Takemoto SK, Zevi A, Feng S, et national conference to assess antibody mediated rejection in solid organ transplantation. Am J Transplant. 2004; 4: 1033-1141.
- Böhmig GA, Wahnmann M, Regele H, et Immunoadsorption in sever C4d positve acute kidney allograft rejection a randomized controlled trial. Am J Transplant. 2001; 7: 117-122.
- Byrne MC, Budisavijevic MN, Fan Z, et el. Renal transplant in patients with Alports syndrome. Am J Kid Dis. 2002; 39: 769-773.
- Bolton WK. Goodpasture s syndrome. Kid Int. 1996; 50: 1753-1759.
- Laczika K, Knapp S, Derfler K, et al. Immunoadsorption in Goodpasture s syndrome. Am J Kid 2000; 36: 392-397.
- Golshayan D, Schwotzer N, Fakhouri F, et al. Targeting the complement pathway in kidney transplantation. J Am Soc Nephrol. 2023; 34:17.
- Dall Amico R, Ghiggeri G, Carraro M, et al. Prediction and treatment of recurrent focal segmental glomerulosclerosis after renal transplantation in children. Am J Kid Dis. 1999; 34: 1048-1055.
- Spasovski C, Trajceska L, Ramnabova Bushljektik I, et Pharmacotherapeutic options for the prevention of kidney transplant rejection the evidence to date. Exp Opin Pharmacother. 2022; 23: 1397-1412.
- Nickerson PW, Böhmig GA, Chadban S, et al. Clazakizumab for the treatment of chronic active antibody mediated rejection in kidney transplant recipients Phas 3 IMAGINE study rationale and design. 2022; 23: 1042.
- Carroll RP, Boyer M, Gebski V, et al. Immune checkpoint inhibitors in kidney transplant recipients a multicenter single arm phase 1 Lancet Oncol. 2022; 23: 1078-1086.
- Yakubu I, Moinuddin I, Gupta G, et al. Use of belatacept in kidney transplantation whats new. Curr Opin Org Transplant. 2023; 28: 36-45.
- Callemeyn J, Lamarthée, Koenig A, et Allorecognition and the spectrum of kidney transplant rejection. Kid Int. 2022; 101: 692-710.
- Ortiz AC, Petrossian G, Addonizio K, et Short term decreased post transplant lymphoproliferative disorder risk after kidney transplantation using two novel regimens. Transplant Immunol. 2023; 76: 101774.
- Shelat Practical considerations for planning a therapeutic apheresis procedure. A m J Med. 2010; 123: 777-784.
- Stefanuti C, Lanfi A, Di Giacomo S, et Therapeutic apheresis in low weight patients: technical feasibility Tolerance compliance and risks. Tranfus Apher. 2004; 19: 417-426.
- Bambauer R, Schiel R, Salgado OJ, et Large bore catheters as vascular access for extracorporeal detoxification methods advantages disadvantages and new improvement. HSOA J Angiol Vasc Surg. 2022; 7: 1000090.
- Wright E, Dillon MJ, Tullus K, et Childhood vasculitis and plasma exchange. Eur J Pediatr. 2007; 166: 145-151.
- Malchesky PS. Therapeutic Apheresis Ther Apher Dial.2015; 19: 417-426.