Therapeutic Use of MicroRNAs to Prevent and Control Allergic Rhinosinusitis
Author'(s): GLADY Gilbert*
President of European Bio Immune(G)ene Medecine Association,Internal medicine, Rue JF Kennedy 1, 68000 Colmar - France.
*Correspondence:
GLADY Gilbert, European Bio Immune(G)ene Medecine Association Europe, 1, Rue JF Kennedy, France, Tel: +33 635 56 21 48; E-mail: info@ebma-europe.com.
Received: 02 January 2018; Accepted: 25 January 2018
Citation: GLADY Gilbert. Therapeutic Use of MicroRNAs to Prevent and Control Allergic Rhinosinusitis. Clin Immunol Res. 2018;2(1): 1-4.
Abstract
Inflammatory upper airway diseases, particularly chronic rhinosinusitis (CRS) and allergic rhinitis (AR), have a high worldwide prevalence. CRS and AR involve sustained and exaggerated inflammation that is associated with marked changes in gene and protein expression under tight regulation. miRNAs represent one of the fundamental epigenetic regulatory mechanisms used by cells that can mediate posttranscriptional gene silencing of target genes. As fine-tuning regulators of gene expression, miRNAs are involved in diverse biological processes, including cell proliferation, apoptosis, and differentiation, organ development, metabolism, stress responses, and signal transduction. Emerging evidence implicates an involvement of miRNAs in shaping the inflammation pattern in upper airways. Studies regarding the roles of miRNAs in allergic diseases have multiplied during the last 4 years, and the functions of miRNAs in the regulation and pathogenesis of these diseases are better and better characterized. Recently, miRNAs have been shown to be detectable in cell-free body fluids such as serum and plasma samples. The circulating miRNAs are protected from blood RNAs either by existing in cell membrane-derived vesicles such as exosomes or by forming a complex with lipid-protein carriers such as high-density lipoprotein. So it becomes possible to use such kind of molecules for a therapeutic purpose, and this is achieved by the Bio Immun(G)en Medicine – BI(G)MED – by introducing high diluted microRNAs in nanocompounds looking for a fine regulation in different upper airways diseases with an allergic aetiology.
Keywords
Introduction
Our objective is to show that it is possible to use the epigenetic regulation induced by microRNAs on the one hand to treat chronic rhinosinusitis of allergic origin, and on the other hand to prevent their occurrence especially seasonal.
Such an approach is made possible by the concepts of endotypes, phenotypes and biomarkers and their interactive effects with the epigenetic mechanisms as described in Figure 1 by Ioana Agache & Cezmi A. Akdis [1].
In this regard, it is necessary to remember that these are diseases expressing a hyperactivity of the TH2 cells causing a disruption of the Th3 / TH2 balance as shown in Figure 2 [2].
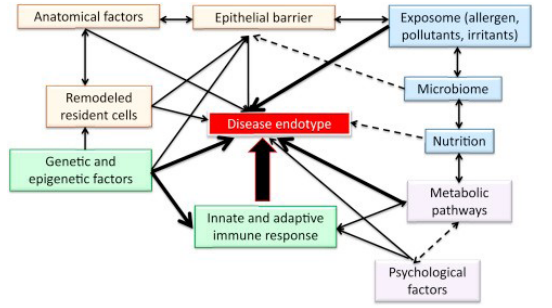
Figure 1: Factors affecting a disease endotype in allergic diseases.
Even though at present time a distinction is made between chronic rhinosinusitis with and without nasal polyps to define so-called endotypes, the latter being more closely related to Th2 cytokines than the former, as shown more recently by Scheckenbach K. and Wagenmann M. in Figure 3 [3].
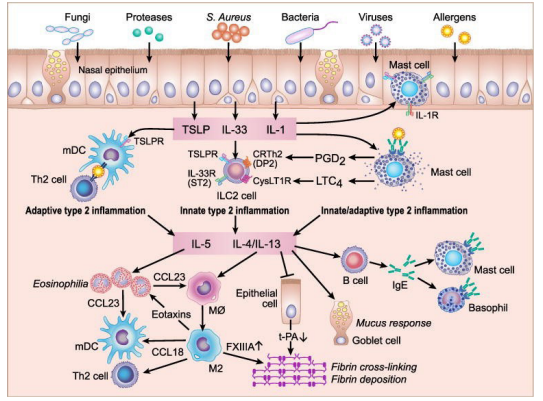
Figure 2: Potential mechanism for amplification of type 2 inflammation in eosinophilic CRSwNP.
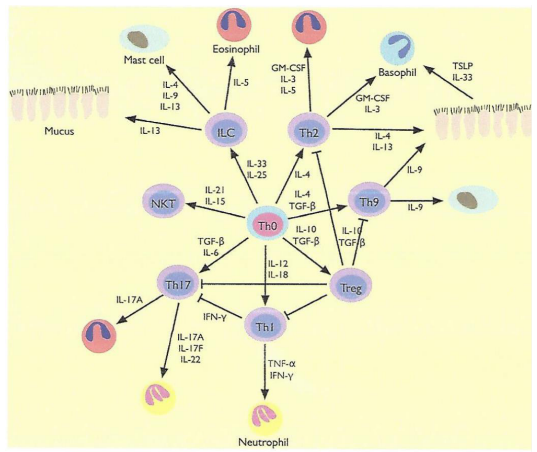
Figure 3: Differentiation of Tcell phenotypes relevant in chronic rhinosinusitis and its different endotypes.
Some years ago, studies have demonstrated that miRNAs are involved in the regulation of allergic inflammation in sinonasal epithelial cells, what allowed Rebane A. & Akdis CA (4) to propose three specific mechanisms by which miRNAs impact allergic inflammation in tissues. First, miRNAs impact the polarization of Th2 cells. Second, miRNAs influence the development and functions of CD8+ Tcells and innate immune cells found in or recruited into the inflamed tissue. Third, miRNAs impact chronic inflammation through their effects in epithelial cells (Figure 4).
At the same time, Lu TX and Rothenberg ME [5], pay particular attention to the regulatory role of microRNAs in relation to a Th3/ TH2 imbalance (Figure 5).
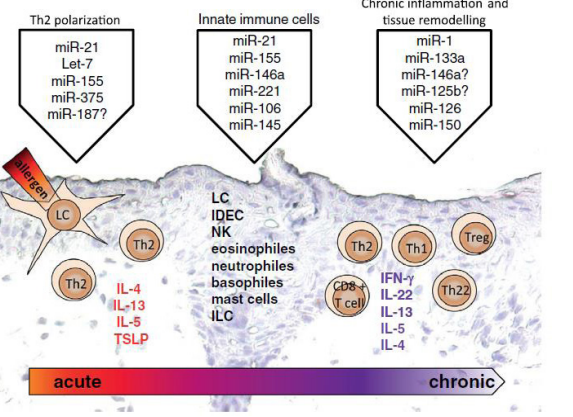
Figure 4: miRNAs in allergic tissue inflammation.
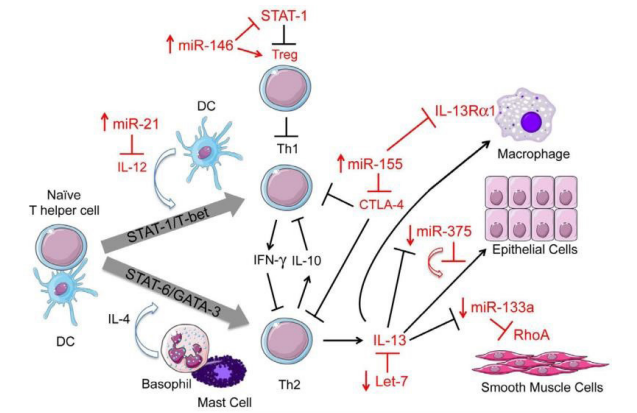
Figure 5: Schematic showing the known roles of a commonly regulated set of miRNAs and their targets in allergic inflammation.
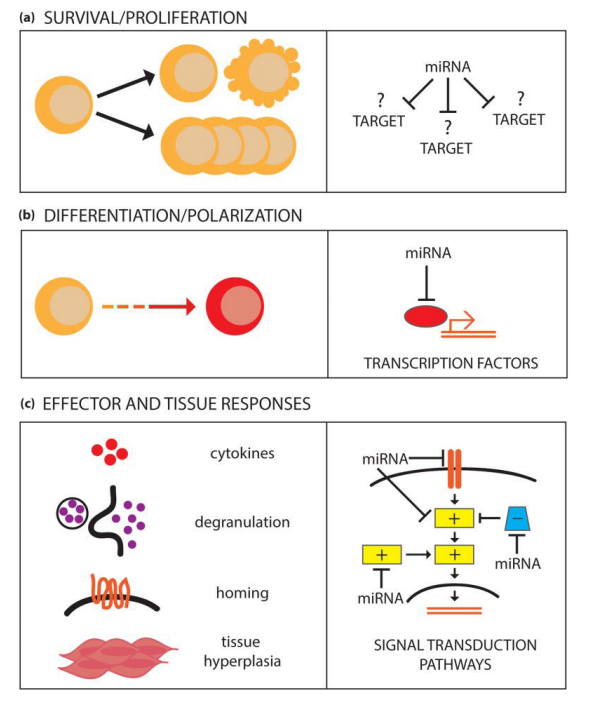
Figure 6: miRNAs regulate multiple facets of type 2 cell function
Pua HH and Ansel KM [6], sometime later focused on the regulatory action of microRNAs more on type 2 cell functions (Figure 6).
At present, it is legitimate to state that microRNAs undoubtedly play a regulatory role in chronic allergic rhinosinusitis by acting on the level of inflammatory reactions taking place in the nasosinus mucosa. These interactions are commonly described in a more general framework, commonly referred to as "United Airway Diseases" [7].
Methods
The aim is to propose a therapeutic use of very weakly concentrated microRNAs, prepared in an appropriate nanotechnological manner and administered sublingually in the context of rhinosinusitis of allergic nature. We will describe a therapeutic formula created for this purpose. To be able to use the microRNAs in a therapeutic way, it is necessary to prepare them appropriately in the form of ultra-low molecular doses by means of high dilutions at levels of 1x10-8 Mol to 1x10-10 Mol, as is possible with modern nanobiotechnologies used in synthetic biology.
To do this, we utilize nanovectors in the form of xylitol globules due to the diffusivity properties of the latter (Figure 7). A huge advantage of using such ultra-low concentrations is the total absence of dangerous side effects.

Figure 7: Xylitol globules used in BI(G)MED-therapy.
The route of administration will necessarily be sublingual in order to benefit from the transmission of the molecular information contained in the xylitol globules to the cells and immunocompetent molecules present in large numbers in the oropharyngeal mucosa.
Results
This nanotechnology has enabled us to develop a drug in the form of two magistral preparations, so-called “BIMUREGs” (for Bio ImMUn REGulators), involving various types of molecules, including a large number of microRNAs (Table I).
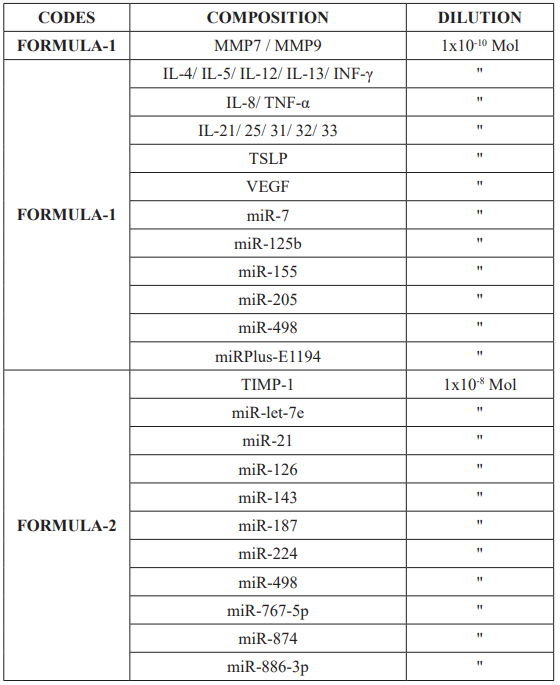
Table 1: Design of the BI(G)MED-formulas involved in allergic rhinosinusitis.
The regulatory effect is obtained by applying the principle of hormesis referring to very low doses, and stipulating that "higher the dilution the greater the effect of inversion of action of a molecule" (Figure 8).
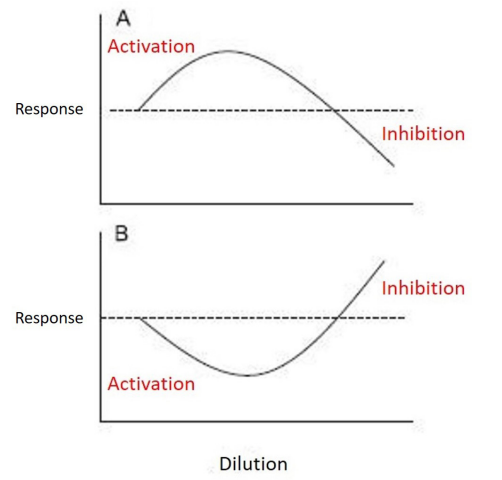
Figure 8: Dose response curve depicting the quantitative features of hormesis.
A short working definition of hormesis is: “a process in which exposure to a low dose of a chemical agent or environmental factor that is damaging at higher doses induces an adaptive beneficial effect on the cell or organism” [8].
The hermetic dose-response is a dose response phenomenon characterized by a low dose stimulation and a high dose inhibition, that may be graphically represented by either an inverted U-shaped dose response or by a J- or U-shaped dose response [9].
This means that a molecule used in a very high dilution (eg 1x10-10 Mol) will not have the same effect as the same molecule used at a lower dilution (eg 1x10-6 Mol), the final effect even reversing gradually as the concentration is lowered.
This emerging concept is more and more often put forward today especially in the context of paradoxical pharmacology, which C. Page sums up very well in the following way:“chronic use of some drug types can have the opposite biological effect(s) to those seen following acute administration of the same drug” [10].
Conclusions
Based on all these theoretical data, we were able to develop a product in the form of a bioimmunological regulator, the use of which proved not only to be very helpful both in the prevention of seasonal allergies and the treatment of chronic rhino-sinusitis of allergic origin but also to be very safe and without side effects. The next step should be that of a clinical trial implemented according to a rigorous double-blind vs. placebo methodology to assess definitively this very innovative therapeutic.
References
- Agache I, Akdis Endotypes of allergic diseases and asthma: An important step in building blocks for the future of precision medicine. Allergol Int. 2016; 65:243-252.
- Kato A. Immunopathology of chronic rhinosinusitis. Allergol 2015; 64: 121-130.
- Scheckenbach K, Wagenmann Cytokine Patterns and Endotypes in Acute and Chronic Rhinosinusitis. Curr Allergy Asthma Rep. 2016; 16: 3.
- Rebane A, Akdis MicroRNAs in allergy and asthma. Curr Allergy Asthma Rep. 2014; 14: 424.
- Lu TX, Rothenberg Diagnostic, functional, and therapeutic roles of microRNA in allergic diseases. J Allergy Clin Immunol. 2013; 132: 3-13.
- Pua HH, Ansel MicroRNA regulation of allergic inflammation and asthma. Curr Opin Immunol. 2015; 36: 101-108.
- Liu Z, Zhang XH, Callejas-Díaz B, et MicroRNA in United Airway Diseases. Int J Mol Sci. 2016; 12: 17.
- Mattson Hormesis defined. Ageing Res Rev. 2008; 7: 1-7.
- Dattilo S, Cesare Mancuso, Guido Koverech, et Heat shock proteins and hormesis in the diagnosis and treatment of neurodegenerative diseases. Immun Ageing. 2015; 12: 20.
- Page Paradoxical pharmacology: turning our pharmacological models upside down. Trends Pharmacol Sci. 2011; 32: 197-200.