Transmissible Cancer: A Canine Transmissible Venereal Tumor during Pregnancy, Case Report
Author'(s): Sergio Ayala-Díaz1, Diego Alberto Vergara Medina2, Marcela Lizano1 and Joaquín Manzo-Merino1,3*
1Unidad de Investigación Biomédica en Cáncer, Instituto Nacional de Cancerología/Instituto de Investigaciones Biomédicas, UNAM.Mexico City, Mexico.
2Departamento de Patología, Facultad de Medicina Veterinariay Zootecnia, UNAM, Mexico City, Mexico.
3CONACyT Research Fellow-Instituto Nacional de Cancerología.Mexico City, Mexico.
*Correspondence:
Joaquín Manzo-Merino, Unidad de Investigación Biomédica en Cáncer, Instituto Nacional de Cancerología/Instituto de Investigaciones Biomédicas, UNAM, Mexico City, Mexico, E-mail:jmanzome@conacyt.mx.
Received: 11 February 2018; Accepted: 03 March 2018
Citation: Sergio Ayala-Díaz, Diego Alberto Vergara Medina, Marcela Lizano, et al. Transmissible Cancer: A Canine Transmissible Venereal Tumor during Pregnancy, Case Report. Cancer Sci Res. 2018; 1(1); 1-4.
Abstract
Canine transmissible venereal cancer (CTVT) is a transmissible cancer affecting dogs worldwide. It has the unique structural and functional capacity to be implanted from a carrier individual to a susceptible one to colonize a new anatomical niche. CTVT establishment depends on several factors including nutritional status, age and whether the dog is in a street situation or not. Here, we describe the CTVT generalities and the clinical management for a pregnant female dog with canine transmissible venereal tumor and propose an algorithm for clinical decision. The outcome of the proposed therapeutic management was the full clearance of the tumor with no relapse.
Keywords
Introduction
Cancer arises when a cell lineage acquires mutations, which can range from single-nucleotide mutations to complete chromosomal changes. This allows the continuous cell proliferation favoring the most prolific sub-clones to survive, which often directs a more specialized genotype and phenotype. Cancer cells can proliferate autonomously, avoid the immune recognition and undergo clonal expansion [1-3]. Particularly, transmissible cancer cells have the structural and functional capacity to be implanted from a carrier individual to a susceptible one where conditions are optimal to colonize a new anatomical niche [4]. Thus, the tumor is transmitted naturally through the eroded mucosa where the epithelial continuity has been broken and where tumor cells that were exfoliated and transplanted to the recipient individual will proliferate. Additionally, among the total number of tumor cells undergoing a transplant, only a 13% will survive until the allograft is established. Karyotypic, genomic, epigenomic and intra-tumoral heterogeneity provide the conditions for individual cancer cells to adapt to the selection pressures imposed by the microenvironment and rapidly acquire new phenotypes, immortalization and/or greater capacity for invasiveness and resistance [2,5].
So far three types of transmissible tumors have been described in mammals, the Canine Transmissible Venereal Tumor (CTVT), the Tasmanian Devil Facial Tumor Disease (DFTD) and a similar tumor of Syrian Hamsters. The CTVT was first identified as a transmissible tumor after the experiments carried out by Novinski in 1876, demonstrating the ability of this tumor of transmitting among individuals of the same species, mainly through direct contact during intercourse [2,4,5]. The CTVT is an unusual pathology in several aspects; first because tumor cells are genetically different from those from the dog since they contain 59 chromosomes, instead of 78 naturally present in dog somatic cells; and secondly, because it is transmitted during the intercourse [6]. The CTVT has an erratic worldwide distribution, occurring more frequently in tropical or subtropical areas, where dogs in street situations have a key role in its dissemination [6]. Although it also occurs with low frequency in purebred animals, due to carelessness and ignorance when mating [7,8].
Genetic evidence suggests that the CTVT cells descend from a common ancestor and once the tumor was generated, it spread in the dog population over time and then two cell lineages were generated extending across the five continents reaching a worldwide distribution [4,9]. Murgia and cols. (2006) performed an analysis searching for 21 canine microsatellite markers and dog leukocyte antigens (DLA) concluding that CTVT cells arose approximately 200 to 2500 years ago in a population of wolves or in some ancient dog breed in East Asia [4]. The study showed that shortly after the emergence of the tumor, the cells underwent drastic genetic changes and they disseminated throughout the canine population with little genetic variation since then. On the other hand, Rebbeck and cols (2009) used microsatellite analysis while conducting a sequencing analysis of the RPPh3 gene and concluded that CTVT is more than 6000 years old. The authors suggested that the origin of the CTVT is restricted to a wolf or a dog [5].
The development of CTVT begins with the implantation of tumor cells in the reproductive system and its consequent exponential growth within two to six months. It can grow slowly and unpredictably for months or years until it reaches stability in size [1,5]. In immunocompetent individuals, the tumor mass tends to retract until it disappears completely. In these individuals, a state of immunity is generated toward the tumor cells, while in immunocompromised individuals the tumor can be invasive and eventually become malignant and metastasize. Also, there are forms of extra-genital presentation which include the oral cavity, nostrils, eyes and skin [8,10,11].
The presumptive diagnosis begins with the physical examination. Clinical signs of the CTVT are presented according to the anatomical location of the tumor mass. When the neoplasm is in the vagina or the penis, it can be detected as it grows and protrudes from the vulva in females, or by the appearance of phimosis in the case of the male. As long as the tumor mass grows, slight hemorrhagic discharges can be seen, which can be confused with urethritis, cystitis or prostatitis. In cases of extra-genital localization, the appearing signs may vary according to the organ location and the definitive diagnosis is based on physical examination and cytology and/or histopathology findings [7,12].
Conventional cytotoxic chemotherapy has been the mainstay for cancer therapy for more than 50 years and its use in pets began early in the 1970s, barely 25 years after the birth of Human Medical Oncology [13]. For both, human and veterinary patients most protocols involve the administration of single or multiple antiproliferative agents administered at doses close to the maximum tolerated dose [14]. For CTVT treatment, several therapeutic alternatives have been used in which the most effective are chemotherapy and radiotherapy, and occasionally surgical treatment [5]. Surgical removal of the primary tumor carries the risk of triggering the proliferation of micro-metastasis and post-treatment recurrence. Nevertheless, its use in the taking of biopsies for diagnosis has a considerable impact on the evolution of the patient by having a timely and accurate diagnosis [8,15,16]. Among the chemotherapeutic agents the Vincristine sulfate has shown the best results, this compound interferes with the assembly of microtubules by combining with tubulin avoiding the cell division and ultimately cell death.
Clinical case
Canine female patient (Canis lupus familiaris), mixed race, adult, was admitted at the reproductive surgery center of the civil association Esteriliza & Educa, from the Perros en Puerto A. C. shelter, Puerto Escondido, México. Physical examination showed good body condition (3/5, on the scale of 1 to 5). Pregnant womb and newly formed tissue was observed in the vagina vestibule, with red, hemorrhagic and irregular edges features, projecting towards the vaginal mucosa with diameters between 4 and 5 centimeters.
A blood count was performed showing mild leukocytosis with granulocytosis and a deviation to the left. Clinical biochemistry analysis showed values within normal parameters for the specie. Microbiological analysis of tumoral tissue of the vagina was assessed by inoculating MacConkey, blood, Sabouraud and chocolate agars. Only few colony forming units (CFU) of Staphylococcus epidermidis were isolated as a single strain. Tissue imprints were made and stained according to the Wright and Papanicolaou technique. Cytological study showed round neoplastic cells with vacuolated eosinophilic cytoplasm, as well as a round hyperchromatic nucleus with one or two evident nucleoli and a large cytoplasm-nucleus relation. Mitosis ranged from 0 t 3A slight anisocytosis and anisokaryosis was appreciated. Among the neoplastic cells, several aggregates of inflammatory cells composed mainly of neutrophils were observed. According to the macroscopic and cellular characteristics described in the different cytological samples (Figure 1), the final diagnosis was Canine Transmissible Venereal Tumor.
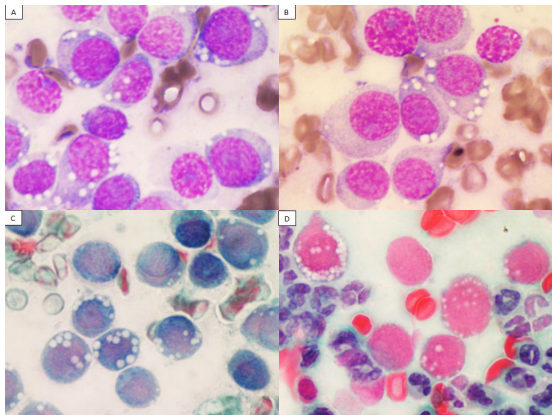
Figure 1: Imprint of newly formed tissue located at the vaginal vestibule. A,B) stained according to the Wright technique, C,D) stained according to the Papanicolaou technique. On a protein background, composed of a moderate amount of well-preserved erythrocytes and neutrophils, neoplastic roving cells are observed, the existing images show moderate amount of eosinophilic cytoplasm, frequently vacuolated. In addition, it shows a slightly eccentric heterochromatic nucleus, with one or more prominent nucleoli.
Since patient showed only few weeks of pregnancy, a hysterectomy with salpingo-oophorectomy was decided by the ethics committee of the civil association Esteriliza & Educa A. C. The procedure was performed and the patient was send to recovery for 21 days and subsequently chemotherapeutic treatment based on Vincristine Sulphate (Crivosin® Vet, Pisa Agropecuaria) was started, at a dosage of 0.023mg/kg for five cycles weekly. One week after starting the treatment, a regression of the tumor mass from approximately 50 to 60% was observed. A cytological monitoring was performed every 30 days for 3 months after the last cycle of chemotherapy, no recurrence was observed during the follow up.
Discussion
CTVT is a highly prevalent neoplasia in tropical and subtropical zones among canine population, mainly in reproductive age [6]. Generally, CTVT exhibits a benign behavior, nevertheless, it can become aggressive and malignant under certain circumstances [2]. This tumor displays a very good response to vincristine based chemotherapeutic treatment [17], but no reports have been generated indicating an approach during pregnancy so far. Thus, we describe here the clinical management of a patient under a pregnancy scenario.
After CTVT confirmed diagnosis, the chemotherapeutic scheme poses a favorable setting for the pregnant mother regarding tumor elimination. Yet, it represents several risks for the developing fetus. In humans, cancer represents the second cause of death among women in reproductive ages and only 0.01 to 0.02% are pregnant, but this data is missing in the veterinarian field [18].
It is worth to mention that pregnancy and cancer are an unusual combination and the information about it is limited. Also, the chemotherapy side effects during pregnancy are associated to an increased risk of teratogenesis and abortions [19,20]. Furthermore, the lack of well established protocol to treat the CTVT/pregnancy binomial delays the beginning of the treatment impacting negatively in the clinical outcome, since treatment decision and evaluation relies mostly in the oncologist experience and in the Ethical committee on duty aimed to cure the patient.
Additionally, the pregnancy stage is critical for clinical decision since during the two first thirds of pregnancy, diagnostics must consider status performance, tumoral size and clinical stage as well as the capacity of the owner to follow to the medical directions before and after treatment. Thus, the recommendation would be the interruption of pregnancy followed by chemotherapy after recovery. Moreover, if patient is diagnosed within the last third of the pregnancy period, in addition to considering the patient’s condition, it is recommended to delay treatment until pregnancy comes to its end naturally. Nonetheless, due to the localization and CTVT characteristics the best option would be a caesarean section with the subsequent formula feeding of the puppies [19] (Figure 2). Whether it is the first or the second situation, surgery followed by hysterectomy with salpingo-oopherectomy or caesarean section is not recommended due to the high probability of recurrency [21].
After gynecological evaluation, our patient underwent hysterectomy and salpingo-oophorectomy. Then, after 21 days of recovery the vincristine-based chemotherapy (Crivosin® Vet, Pisa Agropecuaria) was started following standard regimen at a dosage of 0.023mg/Kg for five cycles weekly. Following the second treatment administration a reduction of 50% was observed, similar to the data reported by Ramirez and cols, with a complete response at cycle five where no evident tumoral mass was detected. Cytological analysis indicated absence of tumoral cells.
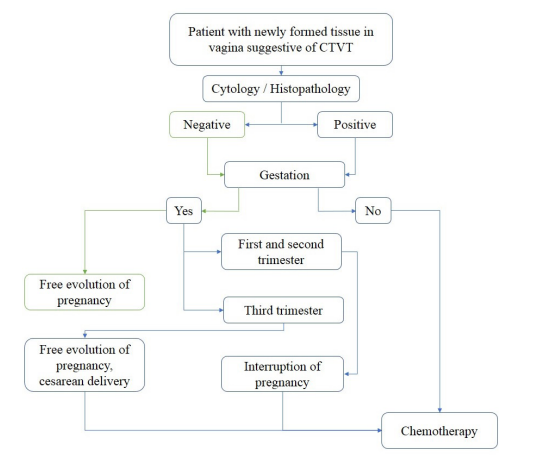
Figure 2: Algorithm in which a route of therapeutic approach in pregnant patients with CTVT is proposed. If the patient is pregnant and their cytology and histopathology are positive to CTVT, the next step is to determine the gestation time, if it is in the first or second trimester of gestation, the interruption of pregnancy is suggested and after the recovery of the surgery, the chemotherapeutic treatment must initiate. If gestation is in the third trimester, it is suggested to continue with the gestation and to schedule a caesarean section, to feed the puppies with milk formula and after the mother recovers, the chemotherapeutic treatment could start.
A cytological follow up was performed for three months after the last chemotherapy cycle and no tumoral cells were observed. Clinical outcome was according to referred data [22,23].
Conclusion
CTVT is frequent among dog population. Although the transmission occurs during the intercourse, the tumoral mass becomes evident only after some months and in case of pregnancy, the product will come to term before the tumor mass can be visualized. Thus, the clinical management in pregnant patients with CTVT requires of an algorithm to give the appropriate therapeutic approach for both the mother and the puppies.
Acknowledgments
We are grateful to the Esteriliza & Educa A. C., Perros en Puerto C. association and to Laboratorio de Diagnóstico Veterinario Especializado from Puerto Escondido, Oaxaca, México for the facilities in the treatment of the patient and a special thanks to Professor Gabriela Ruelas for her contributions.
References
- Birhan G, Chanie A Review on Canine Transmissible Venereal Tumor : from Morphologic to Biochemical and Molecular Diagnosis. Acad J Anim Dis. 2015; 4: 185-195.
- Ujvari B, Papenfuss AT, Belov Transmissible cancers in an evolutionary context. Bio Essays. 2016; 38: 14-23.
- Lee JK, Choi YL, Kwon M, et Mechanisms and Consequences of Cancer Genome Instability: Lessons from Genome Sequencing Studies. Annu Rev Pathol Mech Dis. 2016; 11: 283-312.
- Claudio Murgia, Jonathan Pritchard, Su Yeon Kim, et al. Clonal Origin and Evolution of a Transmissible Cancer Claudio. Cell. 2006; 126: 477-487.
- Ganguly B, Das U, Das AK. Canine transmissible venereal tumour: A Vet Comp Oncol. 2013; 14: 1-12.
- Ortega Pacheco A, Acevedo Arcique M, Sauri Arceo C, et Prevalencia de Tumor Venéreo Transmisible en perros callejeros de la ciudad de Mérida, Yucatán, México. Rev Biomed. 2003; 14: 83-87.
- Romero RR, Ciércoles JAG de J, Garza AMN, et al. Tumor venéreo transmisible con metástasis a un hemangioma esplénico en una Vet Mex. 2010; 41: 305-310.
- Herrera IC, Thomas RE, Carlos Comprobación de la efectividad de dos esquemas terapéuticos en el tratamiento del Tumor de Sticker en perros. Rev Electrónica Vet. 2006; 8: 1-7.
- Strakova A, Leathlobhair MN, Wang GD, et Mitochondrial genetic diversity, selection and recombination in a canine transmissible cancer. E life. 2016; 5: 1-25.
- Rebbeck CA, Thomas R, Breen M, et Origins and evolution of a transmissible cancer. Evolution. 2009; 63: 2340-2349.
- Ojeda J, Alfaro A, Moroni M, et Tumor venéreo transmisible diseminado sobre piel, párpados y pene en un perro. Reporte de caso. Arch Med Vet. 2016; 48: 119-123.
- González CG, Sánchez BCA, Vélez HME, et al. Neoplasias en aparato reproductor en perras: estudio retrospectivo de 6 años. Vet México. 1997; 28:31-34.
- Hantrakul S, Klangkaew N, Kunakornsawat S, et al. Clinical Pharmacokinetics and Effects of Vincristine Sulfate in Dogs with Transmissible Venereal Tumor (TVT). J Vet Med Sci. 2014; 76: 1549-1553.
- Biller Metronomic chemotherapy in veterinary patients with cancer: Rethinking the targets and strategies of chemotherapy. Vet Clin North Am-Small Anim Pract. 2014; 44: 817-829.
- Kadosawa T, Watabe The effects of surgery-induced immunosuppression and angiogenesis on tumour growth. Vet J. 2015; 205: 175-179.
- Boston S, Henderson RA. Role of surgery in multimodal cancer therapy for small animals. Vet Clin North Am-Small Anim 2014; 44: 855-870.
- Özalp G, Zik B, Bastan A, et al. Vincristine modulates the expression of Ki67 and apoptosis in naturally occurring canine transmissible venereal tumor (TVT). Biotech 2012; 87: 325-330.
- M. L. x. Ann Oncol. 2003; 21: 31-36.
- Lataifeh IM, Al masri M, Barahmeh S, et al. Management of Cancer during Pregnancy. Int J Gynecol Cancer. 2011; 21: 1159-1164.
- Cardonick E, Iacobucci Use of chemotherapy during human pregnancy. Lancet Oncol. 2004; 5: 283-291.
- Javanbakht J, Pedram B, Taheriyan MR, et Canine transmissible venereal tumor and seminoma: a cytohistopathology and chemotherapy study of tumors in the growth phase and during regression after chemotherapy. Tumor Biol. 2016; 35: 5493-5500.
- Simon S, Mekha HKK, Antony L. Transmissible venereal tumour in a J Indian Vet Assoc. 2010; 8: 31-34.
- De La Cruz SM, Quijano-Hernández IA, Del Ángel-Caraza J, et Respuesta del Tumor Venéreo Transmisible Canino a Presentaciones de Vincristina de Patente y Genérica. Rev Investig Vet del Peru. 2015; 26: 587-595.