Unique Clinical and Pathologic Characteristics of Poorly Differentiated Primary Pleuro-Pulmonary Synovial Sarcoma
Author'(s): Paul H Hartel, MD1,2,3* and John E. Parker, MD3
1Sligo University Hospital, The Mall, Sligo, Ireland.
2National University of Ireland, Galway School of Medicine,Galway, Ireland.
3West Virginia University School of Medicine, Morgantown, WV,USA
*Correspondence:
Paul H Hartel, MD, Sligo University Hospital, The Mall, Sligo,Ireland F91 H684.
Received: 10 Dec 2021; Accepted: 12 Jan 2022; Published: 16 Jan 2022
Citation: Hartel PH, Parker JE. Unique Clinical and Pathologic Characteristics of Poorly Differentiated Primary Pleuro-Pulmonary Synovial Sarcoma. Cancer Sci Res. 2022; 5(1): 1-5.
Abstract
Primary pleuro-pulmonary synovial sarcoma is rare and poses a diagnostic challenge particularly when poorly differentiated. We present 15 cases of poorly differentiated primary pleuro-pulmonary synovial sarcoma and compare and contrast results with previously published series on usual better differentiated tumors. Clinical and radiologic findings were similar to usual pulmonary synovial sarcoma with typical imaging features. 7 of 10 patients with outcome data died of disease within 5 years. Histologically, tumors had densely cellular fascicles, hyalinized stroma, hemangiopericytoma-like vasculature, and focal myxoid change. Round cell and rare rhabdoid morphology were unique to poorly differentiated tumors. Immunohistochemistry demonstrated at least focal expression of pancytokeratin, EMA, CK7, CK5/6, calretinin, and CD56, and diffuse positivity with CD99 and bcl-2. Ten primary pulmonary synovial sarcomas with tissue available for study were positive for t(x;18), with a more even distribution of SS18/SSX1 and SS18/SSX2 tumors compared with better differentiated lung synovial sarcomas. In conclusion, our study confirms that clinical, histological, and immunohistochemical data from poorly differentiated primary pleuro-pulmonary synovial sarcoma is similar to better differentiated tumours but special caveats remain for accurate histopathologic diagnosis.
Keywords
Introduction
Soft tissue synovial sarcoma is a distinct neoplasm that typically affects deep soft tissues of the extremities in adolescents and young adults [1]. Synovial sarcoma can also present as primary in lung or pleura [2,3]. Primary pleuro-pulmonary synovial sarcoma is an aggressive tumor sharing common histological features with soft tissue synovial sarcoma [3-7]. In approximately 90% of cases, t(x;18) chromosomal translocation will confirm the diagnosis [8]. In t(x;18) negative cases, diagnosis must rely on histological and immunophenotypic features. The differential diagnosis of primary pleuro-pulmonary synovial sarcoma can be challenging when poorly differentiated histologic features are present, particularly on smaller tissue samples. We evaluated the clinical, radiologic, and pathologic findings in 15 cases of poorly differentiated primary pleuro-pulmonary synovial sarcoma and compared our results with better differentiated tumours.
Methods
One hundred and nineteen cases diagnosed as synovial sarcoma or sarcoma, from 1981 to 2019, were retrieved from tissue archives. Fifty-nine cases that failed to meet the World Health Organization histologic and/or immunohistochemical criteria for synovial sarcoma were excluded. Forty-five non-poorly differentiated tumors and metastatic and chest wall tumors were also excluded. Radiologic imaging was available in 5 cases. Follow-up data were obtained from patient records. Hematoxylin and eosin-stained sections were available for each case. Tumors were subtyped as monophasic or biphasic according to World Health Organization
criteria [2] and were all poorly differentiated (Grade 3) following the French Federation of Cancer Centers (FNCLCC) scheme. Immunohistochemistry was performed on paraffin embedded sections using commercially available antibodies (Table 1). Molecular analysis was performed on RNA extracted from paraffin embedded samples. SS18/SSX RNA fusion transcripts resulting from t(x;18)(p11;q11) translocation were detected using real-time reverse transcriptase-polymerase chain reaction [9]. Subtyping of SS18/SSX 1 and 2 fusion transcripts was performed using methods previously described [9].
Results Clinical findings
Clinical features are presented in Table 2. The study group included 8 males and 7 females ranging from 17 to 84 years of age (mean, 41). The most common presenting symptom was shortness of breath. Three of 15 patients had a history of asbestos exposure and 3 were smokers. Tumors were distributed in the lung (9/15) and pleura (6/15). Surgical resection of the primary tumor included pneumonectomy (1), lobectomy (7), and excision of the tumor mass (7). Patients developed local recurrence (2/15) within 21 and 50 months (mean 35). No metastases were reported. Seven of 15 patients died of disease within 6 to 50 months (mean, 17). Two of 15 of patients were alive with no evidence of disease and 1/15 was alive with disease.
Radiologic findings
Radiology included chest radiographs, computed tomography, and magnetic resonance imaging. On chest radiograph tumors appeared well circumscribed with occasional contralateral mediastinal shift. No cavitation, calcification, or lymphadenopathy was identified. On computed tomography, tumors were pleural-based and showed variable enhancement (Figure 1). Ipsilateral pleural effusion was common. Bony destruction or chest wall invasion was not seen. On magnetic resonance, T2-weighted images included high signal intensity suggestive of necrosis, hemorrhage, or myxoid material (Figure 2) and a peripheral rim of enhancement.
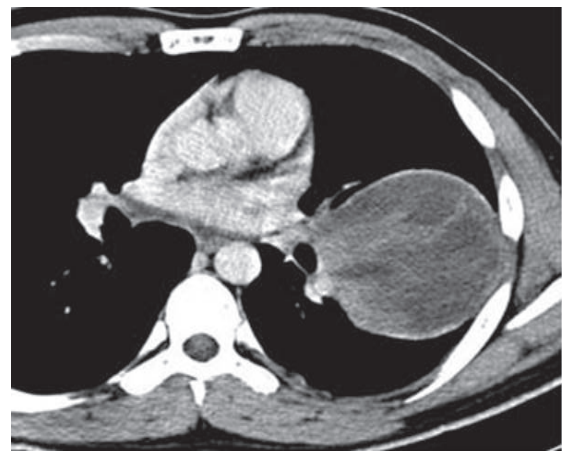
Figure 1: Synovial sarcoma in a young man with chest pain. Contrast- enhanced computed tomography scan shows a cystic mass with peripheral rim enhancement.
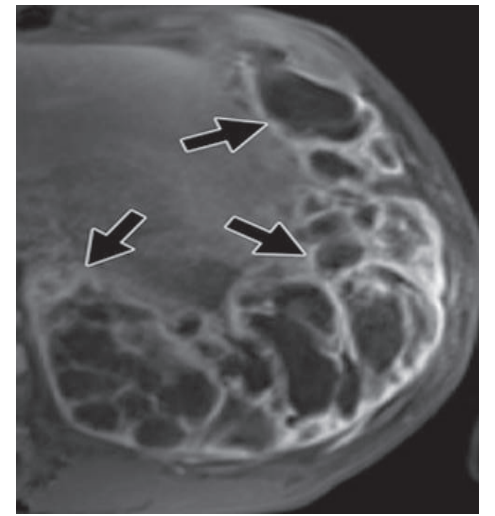
Figure 2: Synovial sarcoma in a young man with dyspnea and pleuritic chest pain. Axial T1-weighted magnetic resonance image shows contrast amongst internal components, with well demarcated cystic spaces (arrows).
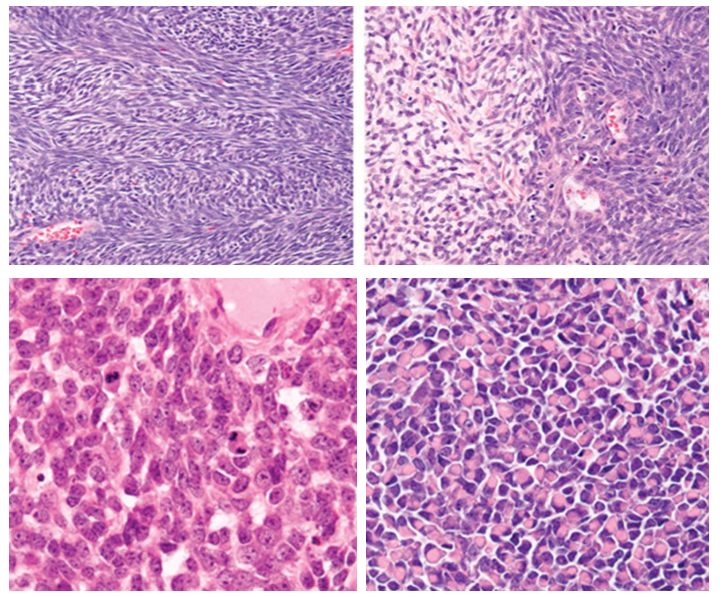
Figure 3: Histologic features of synovial sarcoma including dense spindle cell fascicles and occasional hypocellular myxoid areas(a). Poorly differentiated features that may lead to misdiagnosis include round cell morphology (b) and rhabdoid morphology (c).
Gross and histologic findings
Tumors ranged in size from 5 to19 centimeters (mean, 7.5) and were well-circumscribed, soft, pink-white masses with foci of necrosis, hemorrhage, and cystic change. Histologically, tumors were monophasic (14/15) or biphasic (1/15) and poorly differentiated.
Histological features were those seen with usual pulmonary synovial sarcoma including dense cellularity, interlacing fascicles, hyalinized stroma, hemangiopericytoma-like vasculature, and focal myxoid change. Unique to poorly differentiated tumours were round cell morphology and rhabdoid features (Figure 3)
Mitoses ranged from 18 to 95 per 10 high power fields (mean, 42). Necrosis was present in most tumors (12/15). Benign entrapped pneumocytes were not seen.
Immunohistochemical and molecular findings Immunohistochemical studies showed focal positive staining for epithelial markers (Figure 3) including pancytokeratin, EMA, and CK7. Bcl-2 and CD99 showed diffuse immunoreactivity. CK 5/6, CD56 and calretinin showed focal staining. S-100, smooth muscle actin, desmin and CD34 were negative.
The chromosomal translocation t(x;18) was present in 10 cases with available tissue where 4 were fusion type SS18/SSX1 and 4 were fusion type SS18/SSX2. One case was reported as t(x;18) positive, but information on the SSX fusion type was unavailable. One case was negative.
Conclusion
Synovial sarcoma is a rare primary pleuro-pulmonary neoplasm with distinctive histology. The presence of poorly differentiated histology including round cells and rhabdoid cells may lead to misdiagnosis, particularly with small tissue samples or negative t(x;18) cases. We present 15 poorly differentiated primary pleuro- pulmonary synovial sarcoma cases and compare and contrast our results with literature on better differentiated pleuro-pulmonary synovial sarcoma.
Similar to better differentiated pleuro-pulmonary synovial sarcomas, clinical symptoms are site-specific with rare cases discovered incidentally [4]. Most primary lung synovial sarcomas are located in lung parenchyma [3-7] or pleura. None of our poorly differentiated tumors were mediastinal in origin. Poorly differentiated primary pleuro-pulmonary synovial sarcoma appears more aggressive than better differentiated lung synovial sarcoma, with only 2 patients in this subgroup alive with no evidence of disease in contrast to approximately 30% of patients with better differentiated tumours [10], Twenty percent of our cases had a history of asbestos exposure compared with 6% of better differentiated tumors [10]. Only one case report in the literature discussed asbestos as a possible etiology in synovial sarcoma. Karn et al. [11] reported on a primary cardiac spindle cell tumor with immunoreactivity for cytokeratins and translocation (x;18). Postmortem examination revealed amphibole asbestos within the lungs and diaphragmatic pleural plaques indicative of asbestos exposure. Although no asbestos fibers were seen in tumor or lung tissue in our cases and no pleural plaques were reported on imaging, it is interesting that all of our patients with a history of asbestos exposure had pleural tumors.
Radiologic findings were similar for poorly differentiated pleuro- pulmonary synovial sarcoma as with better differentiated tumors. Tumors show similar areas of tumor, hemorrhage, and necrosis on MRI. On chest radiographs, the lesion is well-circumscribed [12-14] with mediastinal shift in some patients [15]. Computed tomography demonstrates a well-defined enhancing mass with necrosis (Figure 1). Ipsilateral pleural effusion is common [5,12-15], while mediastinal lymphadenopathy is rare [12]. No bone destruction, calcification or invasion into chest wall was seen.
Histologically, our review showed poorly differentiated primary pleuro-pulmonary synovial sarcoma shares identical features with better differentiated tumors including dense cellularity, interlacing fascicles, hyalinized stroma, hemangiopericytoma- like vasculature, and focal myxoid change [3-7], but are unique with foci of round and rhabdoid cell features. Monophasic subtype is most common in usual lung synovial sarcomas, similar to our poorly differentiated tumors. Although we found similar histology with better and poorly differentiated tumors, the round cell and/ or rhabdoid morphology seen with poorly differentiated tumors may be misinterpreted as other primary or metastatic pulmonary neoplasms (Figure 3), particularly with small biopsies or when t(x;18) is negative. Round or epithelioid morphology may be seen in malignant peripheral nerve sheath tumor [16]. The stromal background of malignant peripheral nerve sheath tumor, however, typically appears basophilic and lacks pink stroma. Poorly differentiated primary pleuro-pulmonary synovial sarcoma is often immunoreactive for CK7, while malignant peripheral nerve sheath tumor is negative [17]. Clinically, malignant peripheral nerve sheath tumors arise from nerve or neurofibroma and are associated with neurofibromatosis type I in approximately two-thirds of cases [18].
Poorly differentiated primary pleuro-pulmonary synovial sarcoma may also approximate primitive neuroectodermal tumor. However, primitive neuroectodermal tumor typically has distinct cell borders, clear cytoplasm, scant stroma, and lacks hemangiopericytoma-like vasculature. Both tumors can express CD99, CD56, and cytokeratins [16,19], although expression of CK 7 militates against primitive neuroectodermal tumor [19]. Chromosomal translocation t(11;22) is present in 85% of primitive neuroectodermal tumors [8].
Rhabdoid morphology in poorly differentiated primary pleuro-pulmonary synovial sarcoma may be suggestive of rhabdomyosarcoma, particularly spindle or solid variants of embryonal or alveolar subtypes. Rhabdomyosarcomas are typically immunoreactive with muscle markers. Our cases were negative with smooth muscle actin and desmin. Furthermore, allelic loss in chromosomal region 11p15 and/or t(2;13) can often be detected in rhabdomyosarcoma. While pleomorphic rhabdomyosarcomas have only rarely been shown to harbor chromosomal aberrations that when present are highly complex, the pleomorphic tumor morphology should be helpful in excluding synovial sarcoma.
Poorly differentiated primary pulmonary and pleural synovial sarcoma may be misdiagnosed as carcinoma due to focal round epithelioid cell morphology. Carcinomas typically show greater cytologic atypia with more pleomorphic cytomorphology. Unlike synovial sarcoma, carcinomas may show squamous differentiation or tumor giant cells and diffuse rather than focal cytokeratin positivity. Infiltrative growth and lymph node and systemic metastases are also more common with carcinomas. Radiologic imaging can be helpful. Cytokeratin 5/6 and/or calretinin positivity in synovial sarcoma may raise the possibility of malignant mesothelioma. Solitary pulmonary masses are unusual in mesothelioma which typically has diffuse pleural involvement. Rarely, localized malignant mesothelioma presents as a pleural mass extending into lung parenchyma. In challenging cases, molecular testing for t(x;18) should be helpful.
Our clinicopathological analysis of 15 poorly differentiated primary pleuro-pulmonary synovial sarcoma cases confirmed that this tumor presents with similar clinical and radiologic findings as better differentiated tumors. None of our cases were primary to mediastinum, in contrast to about 11% of better differentiated tumors. All of our patients with a history of asbestos exposure had pleural tumors. Based on our molecular data, poorly differentiated primary pleuro-pulmonary synovial sarcomas can be expected to be negative for t(x;18) by polymerase chain reaction in approximately 6-7% of cases, slightly less than usual better differentiated tumors. SS18/SSX fusion types also appear more evenly distributed. Approximately half of our patients died of disease within 5 years after diagnosis, similar to better differentiated tumors. However, histologically, poorly differentiated primary pleuro-pulmonary synovial sarcoma demonstrated greater mitotic activity and round cell and/or rhabdoid morphology. The latter may lead to misdiagnosis in conjunction with typical demonstration of focal immunoreactivity with at least one epithelial marker. Awareness of poorly differentiated areas in primary pleuro-pulmonary synovial sarcoma and prudent interpretation of immunohistochemistry should prevent diagnostic pitfalls.
References
- Weiss SW, Goldblum JR, Louis, MO, et al. Enzinger and Weiss's Soft Tissue Tumors, Fourth 2001; 1502-1504.
- Travis WD, Brambilla E, Muller-Hermelink HK, et al. World Health Organization Classification of Tumors Pathology and Genetics of Tumors of the Lung Pleura Thymus and Heart IARC Press Lyon France. 2004;
- Zeren H, Moran CA, Suster S, et Primary pulmonary sarcomas with features of monophasic synovial sarcoma a clinicopathological immunohistochemical and ultrastructural study of 25 cases Hum Pathol. 1995; 26: 474-480.
- Begueret H, Galateau-Salle F, Guillou L, et Primary intrathoracic synovial sarcoma a clinicopathologic study of 40 t(x;18)-positive cases from the French Sarcoma Group and the Mesopath Group. Am J Surg Pathol. 2005; 29: 339-346.
- Essary LR, Vargas SO, Fletcher Primary pleuropulmonary synovial sarcoma reappraisal of a recently described anatomic subset. Cancer. 2002; 94: 459-469.
- Okamoto S, Hisaoka M, Daa T, et al. Primary pulmonary synovial sarcoma a clinicopathologic immunohistochemical and molecular study of 11 Hum Pathol. 2004; 35: 850-856.
- Suster S, Moran Primary synovial sarcomas of the mediastinum a clinicopathologic immunohistochemical and ultrastructural study of 15 cases. Am J Surg Pathol. 2005; 29: 569-578.
- Fletcher CD, Unni KK, Mertens World Health Organization Classification of Tumors Pathology and Genetics of Tumors of Soft Tissue and Bone IARC Press Lyon France. 2002; 427.
- Bijwaard KE, Fetsch JF, Przygodzki R, et al. Detection of SYT-SSX fusion transcripts in archival synovial sarcomas by real-time reverse transcriptase-polymerase chain reaction. J Mol 2002; 4: 59-64.
- Hartel PH, Fanburg-Smith JC, Frazier AA, et Primary pulmonary and mediastinal synovial sarcoma A Clinicopathologic study of 60 cases and comparison with 5 prior series. Modern Pathology. 2007; 20: 760-769.
- Karn CM, Socinski MA, Fletcher JA, et al. Cardiac synovial sarcoma with translocation (x;18) associated with asbestos Cancer. 1994; 73: 74-78.
- Duran-Mendicuti A, Costello P, Vargas Primary synovial sarcoma of the chest radiographic and clinicopathologic correlation. J Thorac Imaging. 2003; 18: 87-93.
- Nicholson AG, Goldstraw P, Fisher C. Synovial sarcoma of the pleura and its differentiation from other primary pleural tumours a clinicopathological and immunohistochemical review of three Histopathology. 1998; 33: 508-513.
- Zaring RA, Roepke Pathologic quiz case Pulmonary mass in a patient presenting with a hemothorax Diagnosis primary pulmonary biphasic synovial sarcoma Arch. Pathol Lab Med. 1999; 123: 1287-1289.
- Gaertner E, Zeren EH, Fleming MV, et al. Biphasic synovial sarcomas arising in the pleural cavity a clinicopathologic study of five Am J Surg Pathol. 1996; 20: 36-45.
- Miettinen Diagnostic Soft Tissue Pathology. Churchill Livingstone. New York. 2003; 463-468.
- Smith TA, Machen SK, Fisher C, et Usefulness of cytokeratin subsets for distinguishing monophasic synovial sarcoma from malignant peripheral nerve sheath tumor. Am J Clin Pathol. 1999; 112: 641-648.
- Kleihues P, Cavenee World Health Organization Classification of Tumors Pathology and Genetics of Tumors of the Central Nervous System. 2000; 172-174.
- Machen SK, Fisher C, Gautam RS, et Utility of cytokeratin subsets for distinguishing poorly differentiated synovial sarcoma from peripheral primitive neuroectodermal tumour Histopathology. 1998; 33: 501-507.