Using Haptic Technology for Pain Reduction and Functional Improvement
Author'(s): Jeffrey Gudin1, Janet Fason2,and Peter Hurwitz3*
1University of Miami School of Medicine, Miami, Florida USA.
2Stein Medical Group, Tyrone, Georgia.
3Clarity Science LLC, Narragansett, Rhode Island, USA.
*Correspondence:
Peter Hurwitz, Clarity Science LLC, 750 Boston Neck Road, Suite 11,Narragansett, RI 02882, Tel: +1917 757 0521, Fax: +1855-891-8303.
Received: 22 Dec 2023; Accepted: 28 Jan 2024; Published: 05 Feb 2024
Citation: Gudin J, Fason J, Hurwitz P. Using Haptic Technology for Pain Reduction and Functional Improvement . Anesth Pain Res.2024; 8(1): 1-8.
Abstract
Advancements to reduce pain severity and improve functionality are generally lacking. Chronic or recurrent pain is the most common reason patients consult primary care clinicians. Adverse events associated with existing pharmacological pain treatments have incentivized researchers to identify effective pain treatment strategies that have limited side effects, including non-invasive and non-pharmacologic options. Research has shown that a clearer understanding of the pain neuromatrix may assist in identifying alternative approaches and improving patient outcomes.
A network consisting of neuronal pathways and circuits responding to sensory (nociceptive) stimulation makes up the neuromatrix of pain. Research provides strong support that these pathways and areas of the brain have elicited change in response to external stimuli. Advancements in the understanding of how external tactile stimuli, specifically “haptic vibrotactile trigger technology (VTT)” disrupts the neuromatrix of pain, has led to the development of technology that shows promise in targeting the nociceptive pathways. Through ongoing research, the technology has been incorporated into non-invasive, non-pharmacological topical patches and other routes of delivery to evaluate response as it relates to different health concerns and conditions.
The purpose of this IRB-approved, minimal risk, randomized, and blinded study was to evaluate patients’ experiences and/or perceptions and patient response for those who received a haptic vibrotactile trigger technology (VTT) embedded non-pharmacologic, non-invasive, over-the-counter pain patch (FREEDOM Super Patch with VTT; Srysty Holding CO, Toronto, Canada) and those who received a placebo patch without the embedded technology. This final outcome data from the HARMONI Study adds to previously published interim data.
Methods: Baseline, 7- and 14-day data were recorded in one hundred sixty-eight (168) adult subjects (107 females and 61 males) in a Treatment Group (n=148) or Control Group (n=20) with a mean age of 53 years who presented with mild, moderate and even severe musculoskeletal, arthritic and neurological pain. The study evaluated changes in overall severity and interference scores via a validated scale (Brief Pain Inventory (BPI)), changes in the use of prescription and OTC medications, patient satisfaction, and any side effects reported while using an active or placebo patch.
Results: For the Treatment Group, results showed statistically significant decreases in mean BPI severity and interference scores after using the VTT embedded pain patch. After 14 days, the vast majority of patients reported “less” or “a lot less” usage of oral medications and were very/extremely satisfied with the patch. Results also showed statistically significant and positive outcomes in all measured Quality of Life (QoL) components with improvements in general activity, mood, relations with other people, sleep, normal work, walking ability, and enjoyment of life. In the Control Group, there were no significant changes in pain severity, interference levels, usage of medications, and patient satisfaction was poor during the 14 day study period.
Conclusions: Study results indicate that this non-pharmacologic, non-invasive, haptic vibrotactile trigger technology (VTT) embedded topical patch reduces pain severity and interference scores and may reduce the use of concurrent medications, including prescribed anti-inflammatory and other oral medication for adult patients with arthritic, neuropathic, and musculoskeletal pain. Results reported suggest that the non-pharmacological topical pain patch should be added to the current arsenal of noninvasive and nonpharmacological pain therapies.
Keywords
Introduction
An estimated 100 million people live with pain and, in the United States, pain is the most common reason patients consult primary care providers [1]. There is a reduction in quality of life and impairment on activities of daily living (ADLs) for people experiencing acute and chronic pain, which remain prevalent conditions [2-4].
Due to the potential for serious adverse effects and toxicities of exiting pharmacological pain treatments, researchers have been focused on identifying alternative, less invasive, safe, and effective options that exhibit a reduced side effect profile. As part of a multi-modal approach to care, these less invasive options may provide pain relief without the potential for harmful side effects. In recent years, several medical associations, including the American College of Physicians (ACP) and the American Academy of Family Physicians (AAFP), have updated their guidelines for pain management and recommend a multi-modal approach that includes non-invasive and non-pharmacological therapies as a first line treatment before consideration of other approaches [5,6]. Because of the potential for harmful adverse effects and toxicities, there has been an effort to minimize the use of pharmacologic treatments with the use of other, less harmful alternatives. Ongoing research aims to identify new technologies and treatments that show potential to provide maximum effectiveness, improvement in a patient’s quality of life (QoL), and restore function. Several non-pharmacologic approaches and treatments have been reported to be successful in addressing pain with limited, if any, side effects. These include physical therapeutic, behavioral, and topical drug and device therapies [7-11]. Evidence supports that topical analgesic therapies are safe and effective for pain conditions and should be considered as part of a multi-modal treatment strategy [10-12].
The Gate Control Theory is one of several theoretical frameworks that have been proposed to explain the physiological basis of pain [13]. Through imaging studies, researchers have developed an understanding of how various peripheral, spinal and brain regions modulate and perceive pain [14-16]. Further research has theorized that a network of neuronal pathways and circuits, deemed “the neuromatrix of pain,” responds to sensory (nociceptive) stimulation [14,17,18]. This theory proposes that pain is a multidimensional experience and includes "neurosignature" patterns of nerve impulses generated by a widely distributed neural network in the brain [14,17]. These neurosignature patterns may be triggered by inputs such as tactile sensations. In 2021, the Nobel Prize in Medicine was awarded to 2 scientists for their work in identifying and understanding the roles of different receptors responsible for temperature and touch [18]. Tactile perception is an innate mechanism for human survival and represents our evolved and adaptive ability to apprehend information via haptics – the active touch for object recognition and perception by higher centers of the brain [20,21]. The Nobel scientists identified some important receptors called ion channels. Two of these are named PIEZO1 and PIEZO2 (after píesi, Greek for “pressure”). PIEZO channels have been shown to be involved in our tactile sensation of light touch, pressure, and pain, as well as showing sensitivity to external mechanical stimuli. Piezo2 has essential roles in sensory processes, such as gentle touch sensation [20], This Nobel prize winning work now adds to the growing body of evidence explaining how our bodies sense pain and touch. How pain impulses are generated and therapeutically alleviated are described in a neural network model known as the “neuromatrix” of pain, suggesting that pain originates and is exhibited in specific clusters and patterns [14]. This challenges the “Cartesian” model that theorizes that pain originates in a noxious stimulus resulting from tissue injury or damage [22,23].
The somatosensory experience is determined by a set of channels and receptors sensitive to thermal, tactile, and mechanical stimuli shown to be critical to survival, balance control, and pain modulation [20,21,24]. The application of vibration has long been studied for its analgesic effects. When you get a text or a call on your mobile phone, the vibration you feel is a form of what is called haptic feedback. Haptic feedback systems have been incorporated into prosthetics and other revolutionary medical devices for patients [25-28]. An enhanced technology known as haptic vibrotactile trigger technology (VTT) is designed to target various pathways in your body that connect to the brain centers that control pain, sleep, and anxiety for instance. Researchers have shown that neuronal signals associated with pain can be measured by the electroencephalogram (EEG) [19,29,30]. Using EEG to decode pain perception is an advancement that reveals a spatio-temporal signature associated with pain, nociception, and hyperalgesia. EEG research has shown that haptic vibrotactile trigger technology (VTT) modulates brain centers that are associated with pain pathways [31] in addition to eliciting changes on imaging studies [32].
In this pilot HARMONI (Health Assessments: Reviewing, Measuring, and Observing Neuromatrix Interaction) IRB- approved, minimal risk, observational, non-invasive study, we evaluate an over-the-counter pain-relieving patch (FREEDOM Super Patch with VTT; Srysty Holding Co., Toronto, Canada) that incorporates haptic-vibrotactile trigger technology (VTT) compared with a patch without the embedded technology. The patch is designed and theorized to trigger neural pathways and circuits associated with the neuromatrix of pain and other cortical networks. This study included patients with mild/moderate/severe, and acute or chronic pain and evaluated their overall perceptions of pain treatment and associated symptoms. The Brief Pain Inventory short form (BPI) tool was used to assess patient-reported changes in pain severity and pain interference scores and change in the use of pain medications at 7- and 14-days following treatment. Data presented here are on active treatment (Treatment Group) and non- active treatment (Control Group) and reports on the differences between groups.
Methods Study Design
This study was a prospective, Institutional Review Board-approved Observational Study aimed at evaluating patients’ experiences and/or perceptions and patient response for those who have received a haptic vibrotactile trigger technology (VTT) embedded patch (FREEDOM Super Patch with VTT; Srysty Holding Co., Toronto, Canada) or an inactive pain patch, without embedded VTT technology, by their clinician.
Baseline demographic and clinical characteristics of patients A total of one hundred forty-eight (n=148) patients (96 females, 52 males) at 3 US investigator sites were enrolled in the treatment arm of the study and twenty (n=20) patients (11 females, 9 males) were enrolled in the control group arm of the study. Both groups completed the baseline, day 7, and day 14 surveys. Demographic results were similar for gender and age at the baseline survey for all groups of patients. The mean age at baseline was approximately 53 years for both groups. The primary pain complaint for the patients was recorded at baseline for all groups (Table 1 and Table 2). Myofascial/musculoskeletal pain was the most prominent pain complaint indicated by 54/148 (36.5%) of patients in the Treatment Group and neuropathy/radiculopathy was the most prominent complaint (8/20, 40%) in the control group. Forty-seven (47; 31.8%) patients indicated that neuropathy/radiculopathy and Arthritis was their primary pain complaint in the treatment group.
At baseline, of the 54 study participants in the treatment who indicated myofascial/musculoskeletal pain as their primary complaint, 59% noted that their hips and lower extremities was the most common location of pain (n=33), followed by 39% (n=21) of patients indicating that their neck, back, and shoulders was the area of their pain. Of the remaining 47 patients who indicated arthritis as their primary pain complaint, 81% noted their lower extremities (hip, knee, and foot) was the most common location of their pain (n=38). Almost 30% of patients reported having pain for 3 months to one year (43/148) and over 62% reported having pain for more than one year (93/148). BPI scores indicated that patients receiving the patch embedded with the haptic vibrotactile trigger technology (VTT) were experiencing mild (10%; 15/148), moderate (29%; 43/148), or severe pain (61%; 90/148). In the control group, 15% of patients reported experiencing pain for between 3 months to a year, and 85% reported experiencing pain for at least 1 year. Fifteen percent (15%) of patients reported that they were experiencing moderate pain, and 85% reported that they were experiencing severe pain for at least one year.
Pain management and symptoms were evaluated by patient answers to validated pain measurement and symptom scales (e.g., Brief Pain Inventory (BPI)) as well as additional survey questions regarding patient satisfaction, patient quality of life, and resumption of their normal activities. Evaluation of a Control Group (CG) of patients (given an inactive vehicle patch) is also included in this analysis.
Patients who met the eligibility criteria and who were treated with the pain-relieving patch comprised the study’s treatment group (TG). For the treatment group, patient inclusion criteria were as follows: 1) ages 18 to 85 years, inclusive; 2) ability to provide written informed consent; 3) received the active VTT embedded study patch; and 4) had been diagnosed with a mild/moderate/ severe, acute, or chronic pain condition. Patients who had a history of use drug or alcohol abuse, patients who had an implantable pacemaker, defibrillator or other electrical devices, or patients who were pregnant, were ineligible to participate in the study. For the Control Group, the inclusion and exclusion criteria were the same as the treatment group with the exception that they received a non- active patch that was not embedded with the VTT technology.
Each site provided patients an identification number, and a confidential file containing the informed consent forms and patient identification numbers were kept and maintained in a secured cabinet only accessible to the principal investigator and authorized personnel. Patient survey responses were provided with no identifying patient information. Patients could withdraw from this study at any time with the assurance of no unfavorable impact on their medical care. All diagnostic tests and treatment decisions were made at the discretion of clinicians, with no tests, treatments, or investigations performed as part of this study. Patients were provided the treatment at no cost and were not compensated for their participation in the study.
The study protocol was approved by ADVARRA institutional review board and was performed in full accordance with the rules of the Health Insurance Portability and Accountability Act of 1996 (HIPAA) and the principles of the declaration of Helsinki and the international council of Harmonisation/GCP. All patients gave informed and written consent.
Topical Intervention
The active, non-invasive, 2 x 2-inch non-pharmacological patches are embedded with proprietary sensory pattern imprints and incorporate haptic vibrotactile trigger technology (VTT). The active patches contain no drug or energy source. There is an adhesive backing on one side of the active patch. Patients in the treatment group were instructed to wear one patch near the site of pain and replace the patch each day (Picture 1). The non-active patches look similar to the active patches but do not incorporate the haptic vibrotactile trigger technology (VTT).
Study procedures and assessments
Following enrollment, patients were asked to complete surveys at baseline (day 0) and follow-up on days 7 and 14 of the study period. The surveys were comprised of questions to address and document the nature and location of the primary pain complaint of the patient, which included: 1) arthritis; 2) neuropathy or radiculopathy; or 3) myofascial or musculoskeletal pain. (Locations included neck, shoulders, back, hands, feet, hips, knees, and neck, among others). Study participants indicated only one pain complaint/location, which was the intended patch area for the active and non-active treatment arms.
Included in the survey was the Brief Pain Inventory (BPI), a validated pain assessment tool that is brief and simple to use in both clinical and research settings. This tool assesses not only the severity of pain (0-10 numeric rating scale), but importantly the impact of pain on daily function in patients with cancer pain and other pain conditions [33,34]. We also queried location of pain, pain medications, and amount of pain relief in the past 24 hours or the past week.
For the questions about pain severity, 0 is “no pain” and 10 is “pain as bad as you can imagine.” For the questions about pain interference with activities of daily living, 0 is “does not interfere” and 10 is “completely interferes.” Patient responses to questions regarding pain severity (4 questions) and pain interference (7 questions) were compiled to yield the overall score for pain severity and pain interference.
Patients were asked to indicate any other medications that they had been taking for pain relief at the time of the baseline, day 7, and day 14. Categories of medications that patients could choose included OTC pain medication agents, prescription anti-inflammatory medications, muscle relaxers, opioids, and anticonvulsants. Patients could indicate use of more than one type/ class of analgesic medication.
Study end points
The primary endpoints included changes in patient Brief Pain Inventory (BPI) overall severity and interference scores among and between the treatment group and the control group for the primary pain complaint, as well as changes in the use of prescription and OTC medications. We also assessed patient satisfaction with patch treatment and any side effects reported by patients during the trial.
Statistical analysis
For all variables, descriptive statistics were calculated, including frequencies and percent for categorical variables and means with standard deviation (SD) for continuous variables. The maximum sample size available was used for each statistical analysis.
Changes from baseline to day 7, and to day 14, in BPI mean pain severity and pain interference scores were analyzed using the paired t-test to identify any statistically significant differences within the treatment group, the control group, and between the treatment and control group.
Each survey collected the numbers and types of prescription and OTC oral/topical medications being used for pain relief; statistically significant differences in the use of these types of medications from baseline to day 14 were determined using the McNemar test and χ2 test for binomial paired and unpaired data respectively. Descriptive statistics were used to determine patient satisfaction with the pain-relieving patch within those treated with either the VTT embedded patch or the patch without the VTT technology. Descriptive statistics were also used to report any side effects experienced by patients.
A two-tailed alpha was set to 0.05 for all statistical comparisons. SPSS v. 27 was used for all analyses.
Results
For the Treatment and Control Groups. Paired data were collected; and only patients that completed 14 days of treatment were included in the analysis.
Treatment vs. Control Group
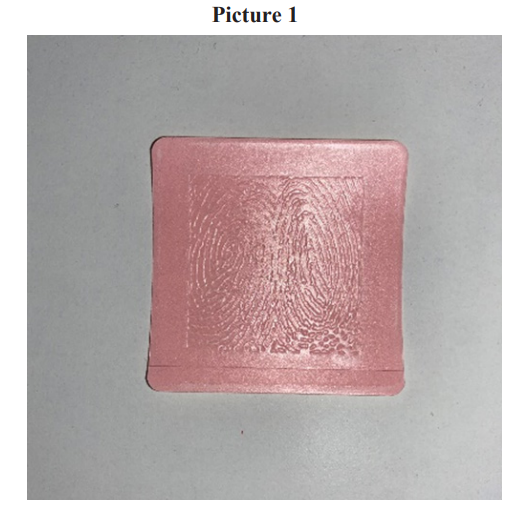
Over 14 days, mean BPI Severity score decreased 47% (4.02 to 2.15/10;P< .001) in the treatment group and 6% (3.66 to 3.45/10;P=.098) in the control group (Table 3 and 4). Mean BPI Interference score decreased 50% (2.59 to 1.29/10;P< .001) in the treatment group and 6% (1.61 to 1.56/10;P= .356) in the control group (Table 5 and 6). After 14 days, in the treatment group, 82% of patients reported “less” or “a lot less” usage of oral medications compared to 5% in the control group. Ninety percent (90%) of patients in the control group reported no change in medication usage over 14 days. In the treatment group, 75% of patients were satisfied with the active patch and of those, 83% were very/ extremely satisfied with the patch. In the control group, all patients were either “not very” satisfied (30%) or “not at all” satisfied (70%) with the inactive patch. In the treatment group, there were statistically significant and positive improvements in light, moderate, or heavy exercise during the 14-day study period (Table 7) and positive outcomes (P<.0.001) in all measured Quality of Life (QoL) components with improvements in general activity,mood, relations with other people, sleep, normal work, walking ability, and enjoyment of life. In the control group, there were no statistically significant changes in any of the QoL components or any noted changes in physical activity levels.
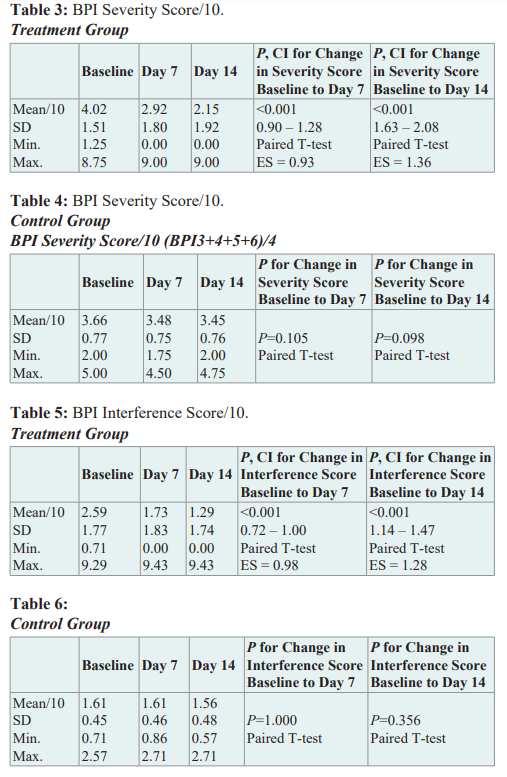
Changes in self-perceived pain relief from medications One of the BPI questions (not part of the pain severity or interference scores) asks the patient how much pain relief (in increments of 10% from 0% = no relief to 100% = complete relief) they have experienced from treatments or medications within the last 24 hours. In the treatment group at baseline, patients reported a mean of 27.6% pain relief from current treatment or medications; by day 7 they reported 61% pain relief, and by day 14 they reported 72% pain relief. The change in mean percent relief from baseline to day 7 was statistically significant (95% CI, 29.2 to 37.9 p < .001) and was also significant from baseline to day 14 (95% CI, 39.2 to 49.4, p < .001). In the control group, there was no change in pain relief from baseline to 7 days (12% to 12%) and only a 2.5% change in pain relief from baseline to day 14 (12% to 14.5%).
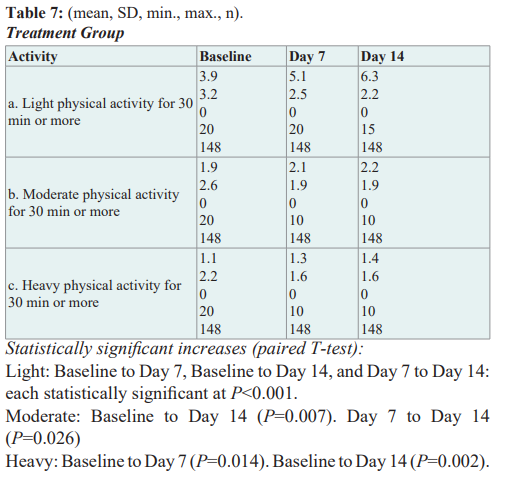
Changes from baseline to day 7 and baseline to day 14 in the use of concurrent pain medications
Patients indicated their utilization of pharmacological treatments for pain at baseline, 7 days, and 14 days. Treatments included OTC agents, prescription anti-inflammatory medications, opioids, anticonvulsants, or muscle relaxants, or a combination of those four classes. At Baseline, there were 51% of patients (85/168) taking an OTC product for their pain, 46% of patients (77/168) taking a prescription anti-inflammatory, 16% (27/168) taking a muscle relaxant, and 2% (3/168) taking an opioid or anticonvulsant.
In the treatment group, there was a decrease in the number of patients using one or more OTC pain medications from Baseline to day 7 and from Baseline to day 14. Approximately 44% of patients reported using Ibuprofen, Naproxen, and/or Acetaminophen at Baseline (65/148). As far as prescription anti-inflammatory medication, naproxen was reported most often 28/148 (19%). At day 14, only 11 patients reported that they were still using a prescription anti-inflammatory. This is a reduction from 79 at Baseline. This is a statistically significant decrease of p <.001. Also noted was a statistically significant decrease in the number of patients using one or more muscle relaxants from Baseline to Day 7 (24 to 2 patients), and Baseline to Day 14 (24 to 1 patient), P<0.001 for each. Although a minority of patients reported using opioids or anticonvulsants at baseline (3, 2%), all but 1 patient discontinued their prescription opioids and anticonvulsants by day 7 which persisted through day 14. In the control group, the 2 patients who reported taking an OTC at baseline were not taking them at day 14. However, there were 8 patients taking a prescription anti-inflammatory medication at baseline, but there was an increase of 2 patients taking this class of medication at day 14.
Use of the Patch
In the treatment group, at day 14, 134/148 (91%) of patients reported that they kept the patch on ‘almost all of the time.’ Of the remaining 14 patients, 6 patients reported that they used the patch ‘until the pain was gone, then again when the pain came back.’
At the first follow-up data collection point at day 7, 82/148 (55%) of patients reported that they felt pain relief in less than 20 minutes after applying the patch. 35% of patients (52/148) reported that it took longer than 20 minutes to feel pain relief. At day 14, 109/148 (74%) of patients reported that they felt pain relief in less than 20 minutes after application and 23/148 (16%) of patients reported pain relief after 20 minutes. In the control group, patients reported that they kept the patch on ‘all of the time” and also reported no pain relief over the 14-day study period.
Duration of Pain Relief
In the treatment group, at day 14, patients were asked how long it took for the pain to return once they removed the patch. Approximately 10% of patients reported that their pain did not return after they removed the patch; 44% of patients (65/148) reported that it took longer than one day for the pain to return after patch removal, and 31/148 (21%) of patients reported that pain was still absent after 2 hours of removing the patch.
Safety
Out of 148 patients in the treatment group, there was only one reported adverse event (local swelling) deemed non-serious by the treating clinician. There were no reports of side effects in the control group.
Discussion
Here we report results of this HARMONI study, a prospective, non-randomized observational study evaluating the safety and analgesic efficacy of the FREEDOM Super Patch with VTT in patients presenting with mild, moderate and even severe musculoskeletal, arthritic and neurological pain. This analysis showed improvements in BPI pain severity and pain interference scores and use of concurrent pain medications from baseline to day 7, and to day 14 in the treatment group and no significant changes or improvement in severity or interference scores in the control group.
Research surrounding haptic vibrotactile trigger technology (VTT) has shown that there is a change in EEG patterns in those patients exposed to VTT [31]. Over the past several years, researchers have developed a better understanding of the Neuromatrix Theory of Pain (NTP) through a broad base of imaging studies and related theories of how different brain regions interact and sense pain [14- 16]. There is a lot of outstanding questions surrounding haptic feedback and its effect on different brain systems. However, researchers continue to explore what appears to be a measurable response to haptic embedded devices [11,23,25,31,32,35].
Chronic pain perception appears to involve multiple neural pathways in addition to those associated with acute pain [17,18]. The networks involved in the perception of painful sensations, as well as their communication and coordination between the CNS and PNS, are broadly referred to as the “neuromatrix” -- the basis for the NTP [14]. Ronald Melzack initially hypothesized that networks of neurons communicating in “large loops”, or through continuous cyclical processing, connect specific regions of the brain with the PNS during sensory processing, deemed the NTP [14]. He envisioned 3 distinct looping pathways: 1) a traditional sensory pathway with neural projections routed through the thalamus, 2) one that follows a path through the brainstem and parts of the limbic system, and 3) one associated with pathways that are routed through different Brodmann Areas (BA), particularly the somatosensory cortex. These loops were meant to explain the cognitive, emotional, and motor modalities through which humans experience sensations, particularly pain [14,15].
Through neuroimaging studies, EEG mapping of the pain neuromatrix is corroborated such as functional analysis using magnetic resonance imaging (fMRI) in many experimental paradigms. The sensory patterns within the patches are in close symmetry between known EEG patterns and their role in modulating EEG and neuronal circuits within higher brain centers. Perceptual, motor, and autonomic responses occupy distinct patterns of the EEG conundrum of pain. It has been shown that pain-related activation of the anterior and posterior cingulate cortices (ACC and PCC, respectively) can lead to identification of various somatosensory circuits associated with proximal and distal sites of the median nerves. This is corroborated by the observations that primary and secondary somatosensory cortices, insular cortex, ACC, prefrontal cortex (PFC), and thalamus are activated centers within the neuromatrix [15]. Eternal stimuli have been shown to influence response in the brain centers targeted by VTT and have produced positive outcomes in balance and stability measurements [35].
There remains an unmet need for alternative treatment options for patients with pain. Potential life-threatening adverse effects have been shown with NSAIDS, acetaminophen, opioids and adjuvant analgesics. Novel, non-pharmacologic and non-invasive therapies fulfill an unmet need for additional safe and effective treatment strategies and options for patients experiencing pain [36-41].
Limitations
This was an IRB-approved observational study based on a sample of patients attending diverse clinical settings for the treatment of arthritic, neurological, and musculoskeletal pain who consented to participate in this study. This analysis reported on a group of 168 patients who were treated with the VTT embedded study patch or a similar looking patch without the embedded VTT technology.
The data of those patients who did not complete the follow up surveys after baseline, or patients who indicated that they did not use the patch after the baseline visit were removed from evaluation. Due to patients having different primary pain complaints and specific location of their pain, overall generalization and consistency of results may be impacted due to the different location of pain, the amount of time the patient utilized the patch, and subjective self-reporting by the patient. We have attempted to accurately evaluate and provide the most detailed reporting of the data while considering these limitations. Although results from the Control Group did not show any significant reductions in BPI severity or interference scores after using the patch without the embedded VTT technology, the number of Control Group patients enrolled and evaluated may have impacted these results. A larger group of Control patients in future studies will assist in confirming the validity of these results due to the nonrandomized nature of this clinical trial. Although there have been many studies about Haptic feedback and the technology associated with haptics, there are still a lot of unknowns as to the precise mechanism of action of how haptics interact with the brain neuro centers.
Conclusion
Study results indicate that this non-pharmacologic, non-invasive, haptic vibrotactile trigger technology (VTT) embedded topical patch reduces pain severity and interference scores and may reduce the use of concurrent medications, including prescribed anti-inflammatory and other oral medication for adult patients with arthritic, neuropathic, and musculoskeletal pain. Further evaluation of control group data supports the effectiveness of this treatment. Results reported here from this IRB-approved observational study suggests that the non-pharmacological topical pain patch embedded with VTT technology should be added to the current arsenal of noninvasive and nonpharmacological pain therapies, including as a first-line non-pharmacological treatment option as part of a multimodal treatment approach.
Acknowledgments
This IRB-approved study administered by Clarity Science LLC was funded by Srysty Holding Co., the distributors of the FREEDOM Super Patch with VTT®.
Disclosure
Jeffrey Gudin MD has received compensation from Clarity Science LLC for his role as principal investigator and for providing protocol-required services for the study. Janet Fason DO received compensation from Clarity Science LLC for her role as a study investigator for the study. Peter L Hurwitz is President of Clarity Science LLC. The authors report no other disclosures.
References
- Finley CR, Chan DS, Garrison S, et al. What are the most common conditions in primary care? Systematic review. Can Fam Physician. 2018; 64: 832-840.
- Jones J, Rutledge DN, Jones KD, et Self-assessed physical function levels of women with fibromyalgia: a national survey. Womens Health Issues. 2008; 18: 406-412.
- Dueñas M, Ojeda B, Salazar A, et A review of chronic pain impact on patients, their social environment and the health care system. J Pain Res. 2016; 9: 457-467.
- Dunsky The Effect of Balance and Coordination Exercises on Quality of Life in Older Adults: A Mini-Review. Front Aging Neurosci. 2019; 11: 318.
- Cuomo A, Bimonte S, Forte CA, et Multimodal approaches and tailored therapies for pain management: the trolley analgesic model. J Pain Res. 2019; 12: 711-714.
- Sharon L Kolasinski, Tuhina Neogi, Marc C Hochberg, et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res (Hoboken). 2020; 72: 149-162.
- Gao YJ, Ji Targeting astrocyte signaling for chronic pain.Neurotherapeutics. 2010; 7: 482-493.
- Cuomo A, Bimonte S, Forte CA, et Multimodal approaches and tailored therapies for pain management: the trolley analgesic model. J Pain Res. 2019; 12: 711-714.
- Gudin JA, Dietze DT, Hurwitz Using Nanotechnology to Improve Pain and Function with a Novel, Drug-Free, Topical Pain-Relief Patch: An Interim Analysis. Anesth Pain Res. 2020; 4: 1-10.
- Gudin JA, Dietze DT, Hurwitz PL. Improvement of Pain and Function After Use of a Topical Pain Relieving Patch: Results of the RELIEF Study. J Pain Res. 2020; 13: 1557-1568.
- Gudin J, Dietze D, Dhaliwal G, et al. Haptic Vibrotactile Trigger Technology: Disrupting the Neuromatrix to Reduce Pain Severity and Interference: Results from the HARMONI Study. Anesth Pain Res. 2022; 6: 1-7.
- Argoff Topical analgesics in the management of acute and chronic pain. Mayo Clin Proc. 2013; 88: 195-205.
- Moayedi M, Davis KD. Theories of pain: from specificity to gate control. J Neurophysiol. 2013; 109: 5-12.
- Melzack R. Pain and the neuromatrix in the brain. J Dent Educ. 2001; 65: 1378-1382.
- Derbyshire SWG. Exploring the pain “neuromatrix.” Curr Rev Pain. 2000; 4: 467-477.
- Mouraux A, Diukova A, Lee MC, et al. A multisensory investigation of the functional significance of the “pain matrix.” Neuroimage. 2011; 54: 2237-2249.
- Weiss Plasticity and cortical reorganization associated with pain. Z Psychol. 2016; 224: 71-79.
- Cheng Y. TRPV1 and Piezo: the 2021 Nobel Prize in Physiology or Medicine. IUCrJ. 2021; 9: 4-5.
- Diers M, Koeppe C, Diesch E, et al. Central processing of acute muscle pain in chronic low back pain patients: an EEG mapping study. J Clin Neurophysiol. 2007; 24: 76-83.
- Fernandes AM, Albuquerque PB. Tactual perception: A review of experimental variables and procedures. Cogn Process. 2012; 13: 285-301.
- Reed CL, Ziat Haptic perception: From the skin to the brain. In Reference Module in Neuroscience and Biobehavioral Psychology. 2018.
- Trachsel LA, Munakomi S, Cascella Pain Theory. Stat Pearls Treasure Island. 2023.
- Wolnei Caumo, Maria Beatriz Cardoso Perioperative anxiety: psychobiology and effects in postoperative recovery. The Pain Clinic. 2003; 15: 87-101.
- Büchel D, Lehmann T, Ullrich S, et Stance leg and surface stability modulate cortical activity during human single leg stance. Exp Brain Res. 2021; 239: 1193-1202.
- Rosenbaum Chou T, Daly Wayne Austin, Ray Chaubey, et Development and Real World Use of a Vibratory Haptic Feedback System for Upper-Limb Prosthetic Users. Journal of Prosthetics and Orthotics. 2016; 4: 28.
- Kim K, Colgate JE. Haptic feedback enhances grip force control of EMG-controlled prosthetic hands in targeted reinnervation IEEE Trans Neural Syst Rehabil Eng. 2012; 20: 798-805.
- Chatterjee A, Chaubey P, Martin J, et al. Testing a prosthetic haptic feedback simulator with an interactive force matching task. J Prosthet Orthot. 2008; 20: 27-34.
- Stepp CE, Matsuoka Y. Vibrotactile sensory substitution for object manipulation: amplitude versus pulse train frequency modulation. IEEE Trans Neural Syst Rehabil Eng. 2012; 20: 31-37.
- Hsueh J, Chen JJ, Shaw F. Distinct somatic discrimination reflected by laser-evoked potentials using scalp EEG leads. Journal of Medical and Biological Engineering. 2016; 36: 460-469.
- LenoirD,WillaertW,CoppietersI,etal.Electroencephalography during nociceptive stimulation in chronic pain patients: a systematic review. Pain Medicine. 2020; 21: 3413-3427.
- Dhaliwal BS, Haddad J, Debrincat M, et al. Changes in Electroencephalogram (EEG) After Foot Stimulation with Embedded Haptic Vibrotactile Trigger Technology: Neuromatrix and Pain Modulation Considerations. Anesth Pain Res. 2022; 6: 1-11.
- Haddad JJ, De Brincat M, North DM, et Cognitive Network Changes After Exposure to Haptic Vibrotactile Trigger Technology: Results From The ENHANCE Study. Neurol Neurosci. 2023; 4: 1-15.
- Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008; 9: 105-121.
- Mendoza TR, Chen C, Brugger A, et al. The utility and validity of the modified brief pain inventory in a multiple-dose postoperative analgesic trial. Clin J Pain. 2004; 20: 357-362.
- Haddad J, Dhaliwal BS, Dhaliwal MS, et Improvement in Balance and Stability Using a Novel Sensory Application: Haptic Vibrotactile Trigger Technology. Int J Res Phys Med Rehabil. 2022; 1: 1-7.
- Farkouh ME, Greenberg An evidence-based review of the cardiovascular risks of nonsteroidal anti-inflammatory drugs. Am J Cardiol. 2009; 103: 1227-1237.
- Harirforoosh S, Jamali Renal adverse effects of nonsteroidal anti-inflammatory drugs. Expert Opin Drug Saf. 2009; 8: 669-681.
- John R, Herzenberg AM. Renal toxicity of therapeutic drugs. J Clin Pathol. 2009; 62: 505-515.
- Lazzaroni M, Porro GB. Management of NSAID-induced gastrointestinal toxicity: focus on proton pump inhibitors. Drugs. 2009; 69: 51-69.
- Scarpignato C, Hunt RH. Nonsteroidal anti-inflammatory drug-related injury to the gastrointestinal tract: clinical picture, pathogenesis, and prevention. Gastroenterol Clin North Am. 2010; 39: 433-464.
- Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ. 2011; 342: 7086.